Nucleotide-Binding Oligomerization Domain 1/Toll-Like Receptor 4 Co-Engagement Promotes Non-Specific Immune Response Against K562 Cancer Cells
- PMID: 35935855
- PMCID: PMC9354050
- DOI: 10.3389/fphar.2022.920928
Nucleotide-Binding Oligomerization Domain 1/Toll-Like Receptor 4 Co-Engagement Promotes Non-Specific Immune Response Against K562 Cancer Cells
Abstract
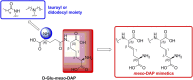
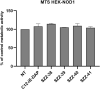
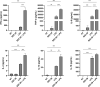
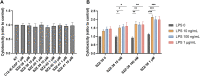
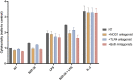
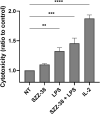
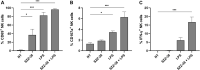
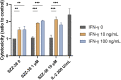
Nucleotide-binding oligomerization domain 1 (NOD1) receptor and Toll-like receptor 4 (TLR4) belong to the family of pattern recognition receptors. Interactions between these receptors profoundly shape the innate immune responses. We previously demonstrated that co-stimulation of peripheral blood mononuclear cells (PBMCs) with D-glutamyl-meso-diaminopimelic acid (iE-DAP)-based NOD1 agonists and lipopolysaccharide (LPS), a TLR4 agonist, synergistically increased the cytokine production. Herein, we postulate that stimulation of NOD1 alone or a combined stimulation of NOD1 and TLR4 could also strengthen PBMC-mediated cytotoxicity against cancer cells. Initially, an in-house library of iE-DAP analogs was screened for NOD1 agonist activity to establish their potency in HEK-Blue NOD1 cells. Next, we showed that our most potent NOD1 agonist SZZ-38 markedly enhanced the LPS-induced cytokine secretion from PBMCs, in addition to PBMC- and natural killer (NK) cell-mediated killing of K562 cancer cells. Activation marker analysis revealed that the frequencies of CD69+, CD107a+, and IFN-γ+ NK cells are significantly upregulated following NOD1/TLR4 co-stimulation. Of note, SZZ-38 also enhanced the IFN-γ-induced PBMC cytotoxicity. Overall, our findings provide further insight into how co-engagement of two pathways boosts the non-specific immune response and attest to the importance of such interplay between NOD1 and TLR4.
Keywords: K562; LPS; NK cells; NOD1 agonist; PBMC; TLR4 agonist; cytolytic activity; synergy.
Copyright © 2022 Guzelj and Jakopin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Synthesis of conformationally constrained γ-D-glutamyl-meso-diaminopimelic acid derivatives as ligands of nucleotide-binding oligomerization domain protein 1 (Nod1).Eur J Med Chem. 2013 Nov;69:232-43. doi: 10.1016/j.ejmech.2013.08.022. Epub 2013 Aug 30. Eur J Med Chem. 2013. PMID: 24044936
-
Nucleotide-binding oligomerization domain protein-1 is expressed and involved in the inflammatory response in human sebocytes.Biochem Biophys Rep. 2023 Oct 27;36:101561. doi: 10.1016/j.bbrep.2023.101561. eCollection 2023 Dec. Biochem Biophys Rep. 2023. PMID: 37942338 Free PMC article.
-
Role of nucleotide-binding oligomerization domain 1 (NOD1) in pericyte-mediated vascular inflammation.J Cell Mol Med. 2016 May;20(5):980-6. doi: 10.1111/jcmm.12804. Epub 2016 Feb 24. J Cell Mol Med. 2016. PMID: 26915562 Free PMC article.
-
Innate Immune Receptors, Key Actors in Cardiovascular Diseases.JACC Basic Transl Sci. 2020 Jul 27;5(7):735-749. doi: 10.1016/j.jacbts.2020.03.015. eCollection 2020 Jul. JACC Basic Transl Sci. 2020. PMID: 32760860 Free PMC article. Review.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
Nucleotide-Binding Oligomerization Domain 1 (NOD1) Agonists Prevent SARS-CoV-2 Infection in Human Lung Epithelial Cells through Harnessing the Innate Immune Response.Int J Mol Sci. 2024 May 13;25(10):5318. doi: 10.3390/ijms25105318. Int J Mol Sci. 2024. PMID: 38791357 Free PMC article.
References
-
- Budikhina A. S., Murugina N. E., Maximchik P. V., Dagil Y. A., Nikolaeva A. M., Balyasova L. S., et al. (2021). Interplay between NOD1 and TLR4 Receptors in Macrophages: Nonsynergistic Activation of Signaling Pathways Results in Synergistic Induction of Proinflammatory Gene Expression. J. Immunol. 206, 2206–2220. 10.4049/jimmunol.2000692 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

