Isoliensinine Suppresses Osteoclast Formation Through NF-κB Signaling Pathways and Relieves Ovariectomy-Induced Bone Loss
- PMID: 35935862
- PMCID: PMC9353689
- DOI: 10.3389/fphar.2022.870553
Isoliensinine Suppresses Osteoclast Formation Through NF-κB Signaling Pathways and Relieves Ovariectomy-Induced Bone Loss
Abstract
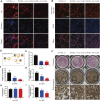
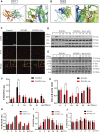
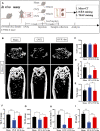
Osteoporosis is among the major contributors of pathologic fracture in postmenopausal women, which is caused by the bone metabolic disorder owing to the over-activation of osteoclasts. Inhibition of osteoclast differentiation and maturation has become a mainstream research interest in the prevention of osteoporosis. Isoliensinine (Iso) is a dibenzyl isoquinoline alkaloid with antioxidant, anti-inflammatory, and anti-cancer activities. However, whether it can be used as a potential treatment for osteoporosis remains undiscovered. Here, we investigated whether Iso might suppress the differentiation of osteoclasts in vitro and in vivo to play an anti-osteoporosis role. Our results showed that Iso inhibits the formation of mature multinuclear osteoclasts induced by RANKL, the bone resorption, and the osteoclast-specific genes expression by blocking the nuclear translocation of NF-κB p65, and the effect was in a dosage-dependent way. Furthermore, we investigated the therapeutic effect of Iso on osteoporosis in ovariectomized (OVX) mice. We found that Iso attenuated bone loss in the OVX mice and significantly promoted BS, Conn. DN, Tb.Th, TB.N, and BV/TV Index. All in all, Iso showed a prominent effect of osteoclast inhibition, with great promise for treating osteoporosis.
Keywords: NF-κB pathway; isoliensinine; osteoclast; osteoporosis; receptor activator of nuclear factor-κB ligand (RANKL).
Copyright © 2022 Liu, Gu, Huang, Liu, Liu, Liao, Feng, Xie, Zhao, Xu, Liu and Zhan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways.J Ethnopharmacol. 2021 Aug 10;276:114176. doi: 10.1016/j.jep.2021.114176. Epub 2021 Apr 30. J Ethnopharmacol. 2021. PMID: 33933570
-
Tetrandrine Attenuates Cartilage Degeneration, Osteoclast Proliferation, and Macrophage Transformation through Inhibiting P65 Phosphorylation in Ovariectomy-induced Osteoporosis.Immunol Invest. 2022 Apr;51(3):465-479. doi: 10.1080/08820139.2020.1837864. Epub 2020 Nov 3. Immunol Invest. 2022. PMID: 33140671
-
Hydrogen gas protects against ovariectomy-induced osteoporosis by inhibiting NF-κB activation.Menopause. 2019 Jul;26(7):785-792. doi: 10.1097/GME.0000000000001310. Menopause. 2019. PMID: 31083022
-
Melatonin Inhibits Osteoclastogenesis and Bone Loss in Ovariectomized Mice by Regulating PRMT1-Mediated Signaling.Endocrinology. 2021 Jun 1;162(6):bqab057. doi: 10.1210/endocr/bqab057. Endocrinology. 2021. PMID: 33713122
-
How zoledronic acid improves osteoporosis by acting on osteoclasts.Front Pharmacol. 2022 Aug 25;13:961941. doi: 10.3389/fphar.2022.961941. eCollection 2022. Front Pharmacol. 2022. PMID: 36091799 Free PMC article. Review.
Cited by
-
Myrislignan targets extracellular signal-regulated kinase (ERK) and modulates mitochondrial function to dampen osteoclastogenesis and ovariectomy-induced osteoporosis.J Transl Med. 2023 Nov 22;21(1):839. doi: 10.1186/s12967-023-04706-2. J Transl Med. 2023. PMID: 37993937 Free PMC article.
-
Research progress on pharmacological effects and mechanisms of cepharanthine and its derivatives.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2843-2860. doi: 10.1007/s00210-023-02537-y. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338575 Review.
References
-
- Aliprantis A. O., Ueki Y., Sulyanto R., Park A., Sigrist K. S., Sharma S. M., et al. (2008). NFATc1 in Mice Represses Osteoprotegerin during Osteoclastogenesis and Dissociates Systemic Osteopenia from Inflammation in Cherubism. J. Clin. Invest. 118 (11), 3775–3789. 10.1172/jci35711 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

