The human cathelicidin peptide LL-37 inhibits pancreatic cancer growth by suppressing autophagy and reprogramming of the tumor immune microenvironment
- PMID: 35935871
- PMCID: PMC9355328
- DOI: 10.3389/fphar.2022.906625
The human cathelicidin peptide LL-37 inhibits pancreatic cancer growth by suppressing autophagy and reprogramming of the tumor immune microenvironment
Abstract
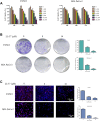
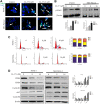
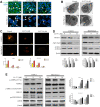
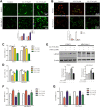
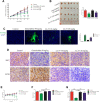
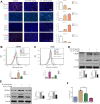
Pancreatic cancer is amongst the most lethal malignancies, while its poor prognosis could be associated with promotion of autophagy and the tumor immune microenvironment. Studies have confirmed the pro-tumorigenic nature of the cathelicidin family of peptide LL-37 in several types of cancer. However, at higher doses, LL-37 exerts significant cytotoxicity against gastrointestinal cancer cells. In our study, we investigated the anti-tumorigenic potential of LL-37 in pancreatic cancer and the underlying mechanisms. Our results have shown that LL-37 inhibited the growth of pancreatic cancer both in vitro and in vivo. Mechanistic studies have demonstrated that LL-37 induced DNA damage and cell cycle arrest through induction of reactive oxygen species (ROS). Further study indicates that LL-37 suppressed autophagy in pancreatic cancer cells through activation of mTOR signaling, leading to more accumulation of ROS production and induction of mitochondrial dysfunctions. With combined treatment of LL-37 with the mTOR inhibitor rapamycin, LL-37-induced ROS production and cancer cell growth inhibition were attenuated. Subsequent in vivo study has shown that LL-37 downregulated the immunosuppressive myeloid-derived suppressor cells and M2 macrophages while upregulated the anti-cancer effectors CD8+ and CD4+ T cells in the tumor microenvironment. By using an in vitro co-culture system, it was shown that promotion of M2 macrophage polarization would be suppressed by LL-37 with inhibition of autophagy, which possessed significant negative impact on cancer growth. Taken together, our findings implicate that LL-37 could attenuate the development of pancreatic cancer by suppressing autophagy and reprogramming of the tumor immune microenvironment.
Keywords: LL-37; ROS; autophagy; mTOR signaling; pancreatic cancer; tumor immune microenvironment.
Copyright © 2022 Zhang, Chen, Zhang, Bai, Lau, Sze, Yung and Ko.
Conflict of interest statement
The handling editor YB declared a shared affiliation with the author S-QZ at the time of review. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bang S., Kim H. S., Choo Y, S., Park S W., Chung J, B., Song S, Y., et al. (2006). Differences in immune cells engaged in cell-mediated immunity after chemotherapy for far advanced pancreatic cancer. Pancreas 32 (1), 29–36. 10.1097/01.mpa.0000191651.32420.41 PubMed Abstract | 10.1097/01.mpa.0000191651.32420.41 | Google Scholar - DOI - PubMed
-
- Carriere A., Romeo Y., Acosta-Jaquez H. A., Moreau J., Bonneil E., Thibault P., et al. (2011). ERK1/2 phosphorylate raptor to promote ras-dependent activation of MTOR complex 1 (MTORC1). J. Biol. Chem. 286 (1), 567–577. 10.1074/jbc.M110.159046 PubMed Abstract | 10.1074/jbc.M110.159046 | Google Scholar - DOI - PMC - PubMed
-
- Chen D., Xie J., Fiskesund R., Dong W., Liang X., Lv J., et al. (2018). Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 9 (1), 873. 10.1038/s41467-018-03225-9 PubMed Abstract | 10.1038/s41467-018-03225-9 | Google Scholar - DOI - PMC - PubMed
-
- Chen M. L., Pittet M. J., Gorelik L., Flavell R. A., Weissleder R., Von Boehmer H., et al. (2005). Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo . Proc. Natl. Acad. Sci. U. S. A. 102 (2), 419–424. 10.1073/pnas.0408197102 PubMed Abstract | 10.1073/pnas.0408197102 | Google Scholar - DOI - PMC - PubMed
-
- Chen X., Qi G., Qin M., Zou Y., Zhong K., Tang Y., et al. (2017). DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity. Oncotarget 8 (17), 27943–27952. 10.18632/oncotarget.15847 PubMed Abstract | 10.18632/oncotarget.15847 | Google Scholar - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

