Peripheral Neuroprotective and Immunomodulatory Effects of 5α-Reductase Inhibitors in Parkinson's Disease Models
- PMID: 35935876
- PMCID: PMC9355275
- DOI: 10.3389/fphar.2022.898067
Peripheral Neuroprotective and Immunomodulatory Effects of 5α-Reductase Inhibitors in Parkinson's Disease Models
Abstract
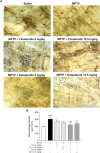
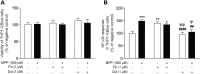
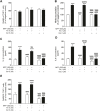
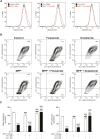
Gastrointestinal disorders in Parkinson's disease (PD) have been associated with neuronal alteration in the plexus of the gut. We previously demonstrated the immunomodulatory effect of female hormones to treat enteric neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. This study made the hypothesis of obtaining similar neuroprotection as with hormone treatments by affecting steroidogenesis with two 5α-reductase inhibitors, finasteride and dutasteride. These drugs are approved to treat benign prostatic hyperplasia and alopecia and display mitochondrial effects. In MPTP-treated mice, the dopaminergic and vasoactive intestinal peptide (VIP) neurons alteration was prevented by finasteride and dutasteride, while the increase in proinflammatory macrophages density was inhibited by dutasteride treatment but not finasteride. NF-κB response, oxidative stress, and nitric oxide and proinflammatory cytokines production in vitro were only prevented by dutasteride. In addition, mitochondrial production of free radicals, membrane depolarization, decreased basal respiration, and ATP production were inhibited by dutasteride, while finasteride had no effect. In conclusion, the present results indicate that dutasteride treatment prevents enteric neuronal damages in the MPTP mouse model, at least in part through anti-inflammatory and mitochondrial effects. This suggests that drug repurposing of dutasteride might be a promising avenue to treat enteric neuroinflammation in early PD.
Keywords: MPTP; dutasteride; enteric nervous system; female hormones; finasteride; gut; inflammation; mitochondria.
Copyright © 2022 Poirier, Côté, Jarras, Litim, Lamontagne-Proulx, Al-Sweidi, Morissette, Lachhab, Pelletier, Di Paolo and Soulet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

