Effect of Acid Suppressants on Non- Helicobacter pylori Helicobacters Within Parietal Cells
- PMID: 35935877
- PMCID: PMC9355715
- DOI: 10.3389/fphar.2022.692437
Effect of Acid Suppressants on Non- Helicobacter pylori Helicobacters Within Parietal Cells
Abstract
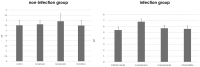
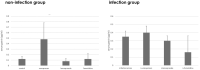
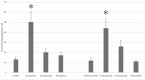
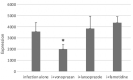
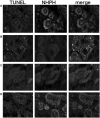
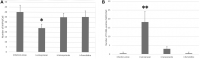
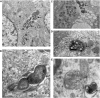
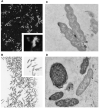
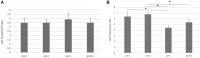
We investigated the effect of increased pH induced by acid suppressants on the viability of non-Helicobacter pylori helicobacters (NHPHs) within parietal cell intracellular canaliculi and fundic glandular lumina by immunohistochemistry, electron microscopy, quantitative PCR, urea breath tests, and using a bilayer culture system. Three months before the experiment, mice were infected with the NHPH H. suis and then treated with famotidine (2 mg/kg body weight [BW], once daily), lansoprazole (30 mg/kg BW, once daily), or vonoprazan (20 mg/kg BW, once daily) for 3 days. Immunohistochemical studies using the TUNEL method, quantitative PCR analysis, and urea breath tests were performed. PCR analysis showed a decrease in the NHPH quantity after vonoprazan treatment. Urea breath tests revealed a significant decrease in the NHPH urease activity after vonoprazan, lansoprazole, and famotidine treatments for 3 days; however, 4 days after the treatment, urease activity reversed to the pretreatment level for each treatment group. Electron microscopy revealed an increase in the damaged NHPH after vonoprazan treatment. The TUNEL method revealed apoptotic NHPH within parietal cells after vonoprazan treatment. The bilayer culture results demonstrated that NHPH moved more quickly at a pH of 4.0 than at a pH of 3.0, 5.0, and 6.5, and electron microscopy revealed a change from the spiral form to the coccoid form under near-neutral pH conditions. We thus proposed that acid suppressants, especially vonoprazan, induce NHPH damage by altering pH.
Keywords: Helicobacter suis; TUNEL; bilayer culture; coccoid form; non-Helicobacter pylori helicobacter; urea breath test; vonoprazan.
Copyright © 2022 Nakamura, Murasato, Øverby, Kodama, Michimae, Sasaki, Flahou, Haesebrouck, Murayama, Takahashi, Uchida, Suzuki and Matsui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
PCR analysis and specific immunohistochemistry revealing a high prevalence of non-Helicobacter pylori Helicobacters in Helicobacter pylori-negative gastric disease patients in Japan: High susceptibility to an Hp eradication regimen.Helicobacter. 2020 Oct;25(5):e12700. doi: 10.1111/hel.12700. Epub 2020 Aug 13. Helicobacter. 2020. PMID: 32790220
-
Prevalence, clinical features, and esophagogastroduodenoscopy (EGD) findings of non-Helicobacter pylori Helicobacter infection: A study of 50 cases at a single facility in Japan.Helicobacter. 2021 Aug;26(4):e12811. doi: 10.1111/hel.12811. Epub 2021 Apr 27. Helicobacter. 2021. PMID: 33908121
-
Effect of Vonoprazan, a Potassium-Competitive Acid Blocker, on the 13C-Urea Breath Test in Helicobacter pylori-Positive Patients.Dig Dis Sci. 2017 Mar;62(3):739-745. doi: 10.1007/s10620-016-4439-0. Epub 2017 Jan 12. Dig Dis Sci. 2017. PMID: 28083842 Clinical Trial.
-
Gastric and enterohepatic non-Helicobacter pylori Helicobacters.Helicobacter. 2013 Sep;18 Suppl 1:66-72. doi: 10.1111/hel.12072. Helicobacter. 2013. PMID: 24011248 Review.
-
Non-Helicobacter pylori helicobacters detected in the stomach of humans comprise several naturally occurring Helicobacter species in animals.FEMS Immunol Med Microbiol. 2009 Apr;55(3):306-13. doi: 10.1111/j.1574-695X.2009.00535.x. Epub 2009 Feb 19. FEMS Immunol Med Microbiol. 2009. PMID: 19243435 Review.
Cited by
-
Development of serological assays to identify Helicobacter suis and H. pylori infections.iScience. 2023 Mar 29;26(4):106522. doi: 10.1016/j.isci.2023.106522. eCollection 2023 Apr 21. iScience. 2023. PMID: 37123222 Free PMC article.
-
Triple-drug combination therapy versus six-month proton pump inhibitor monotherapy in non-Helicobacter pylori Helicobacter eradication, and hyperacid environment preference of Helicobacter suis: a clinical study.BMC Gastroenterol. 2024 May 8;24(1):157. doi: 10.1186/s12876-024-03252-5. BMC Gastroenterol. 2024. PMID: 38720287 Free PMC article.
References
-
- Bizzozero G. (1893). Ueber die schlauchförmigen Drüsen des Magendarmkanals und die Beziehungen ihres Epithels zu dem Oberflächenepithel der Schleimhaut Dritte Mittheilung. Archiv f. mikrosk. Anat. 42, 82–152. 10.1007/bf02975307 - DOI
LinkOut - more resources
Full Text Sources

