The Impact of Proton Pump Inhibitors on the Development of Gastric Neoplastic Lesions in Patients With Autoimmune Atrophic Gastritis
- PMID: 35935934
- PMCID: PMC9353125
- DOI: 10.3389/fimmu.2022.910077
The Impact of Proton Pump Inhibitors on the Development of Gastric Neoplastic Lesions in Patients With Autoimmune Atrophic Gastritis
Abstract
Introduction: Proton pump inhibitors (PPIs) have been widely prescribed as a primary treatment for acid-related disorders. A large body of literature reported several adverse outcomes due to PPI therapy, including an increased risk of gastric cancer (GC). Autoimmune atrophic gastritis (AAG) is a chronic inflammatory disorder affecting the oxyntic mucosa, leading to mucosal atrophy, intestinal metaplasia, and reduced gastric acid secretion, up to the possible development of dysplasia and intestinal-type GC. Whether PPI use may increase the GC risk in AAG patients has not yet been investigated. We conducted a case-control study in AAG patients to assess the association between the PPI use before AAG diagnosis and the development of GC at follow-up (FU).
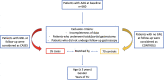
Materials and methods: Patients were included from a prospective cohort of AAG patients (diagnosed 1992-2021) in a referral center for gastric autoimmunity; all patients adhered to an endoscopic-histological FU program according to Management of precancerous conditions and lesions in the stomach (MAPS) I/II (management of epithelial precancerous conditions) guidelines. At diagnosis, clinical/biochemical data and PPI use before AAG diagnosis (withdrawn at the time of diagnosis), for at least 12 months, were evaluated. Patients who developed gastric neoplastic lesions (GNLs) at FU were considered as cases; patients without a diagnosis of GNLs at FU were considered as controls. At a total FU of 2.3 years (1-13), 35 cases were identified, and controls were matched 2:1 by age ( ± 3 years), gender, and years of FU (n=70); therefore, a total of n=105 patients were included in the study.
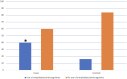
Results: The proportion of PPI users before AAG diagnosis was significantly higher in cases than in controls (54.3% vs. 18.6%, p<0.001). At logistic regression, considering as a dependent variable the development of GNLs at FU, a positive association was shown for PPI use before AAG diagnosis (OR 9.6, 95%CI 2.3-40.3), while other independent variables as the use of antiplatelets/anticoagulants (OR 2.8, 95%CI 0.7-12.0), age ≥ 50 years (OR 2.0, 95%CI 0.2-18.1), 1st-degree family history for GC (OR 2.4, 95%CI 0.4-15.2), and smoking habit (OR 0.4, 95%CI 0.1-2.1) were not associated.
Conclusions: PPI use before the diagnosis of AAG appears to considerably increase the risk of subsequent GNL development. Considering the common misuse of PPIs, physicians should regularly reevaluate the appropriateness of ongoing PPI therapy, in particular in patients with a clinical suspicion of or already diagnosed AAG.
Keywords: autoimmune atrophic gastritis; gastric autoimmunity; gastric cancer; gastric neoplastic lesion; proton pump inhibitor (PPI).
Copyright © 2022 Dilaghi, Bellisario, Esposito, Carabotti, Annibale and Lahner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Autoimmune chronic atrophic gastritis: association between chronic proton pump inhibitors use and more severe atrophy and gastric intestinal metaplasia.Eur J Gastroenterol Hepatol. 2025 Aug 1;37(8):905-910. doi: 10.1097/MEG.0000000000002989. Epub 2025 Jun 25. Eur J Gastroenterol Hepatol. 2025. PMID: 40470694 Free PMC article.
-
Effects of Proton Pump Inhibitor on the Distribution of Helicobacter pylori and Associated Gastritis in Patients with Gastric Atrophy.Digestion. 2020;101(3):279-286. doi: 10.1159/000499424. Epub 2019 May 8. Digestion. 2020. PMID: 31067538
-
Gastric neuroendocrine tumours from long-term proton pump inhibitor users are indolent tumours with good prognosis.Histopathology. 2020 Dec;77(6):865-876. doi: 10.1111/his.14220. Epub 2020 Sep 20. Histopathology. 2020. PMID: 32702178
-
Effect of long-term proton pump inhibitor administration on gastric mucosal atrophy: A meta-analysis.Saudi J Gastroenterol. 2017 Jul-Aug;23(4):222-228. doi: 10.4103/sjg.SJG_573_16. Saudi J Gastroenterol. 2017. PMID: 28721975 Free PMC article. Review.
-
Gastric atrophy, metaplasia, and dysplasia: a clinical perspective.J Clin Gastroenterol. 2003 May-Jun;36(5 Suppl):S29-36; discussion S61-2. doi: 10.1097/00004836-200305001-00006. J Clin Gastroenterol. 2003. PMID: 12702963 Review.
Cited by
-
A Systematic Review of the Adverse Effects of Long-Term Proton Pump Inhibitor Use on the Gastrointestinal System in the Adult Population.Cureus. 2025 Aug 20;17(8):e90606. doi: 10.7759/cureus.90606. eCollection 2025 Aug. Cureus. 2025. PMID: 40843060 Free PMC article. Review.
-
Proton pump inhibitors and the risk of gastric cancer: a systematic review, evidence synthesis and life course epidemiology perspective.BMJ Open Gastroenterol. 2025 Apr 19;12(1):e001719. doi: 10.1136/bmjgast-2024-001719. BMJ Open Gastroenterol. 2025. PMID: 40253055 Free PMC article.
-
Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis.World J Gastrointest Oncol. 2023 Aug 15;15(8):1451-1460. doi: 10.4251/wjgo.v15.i8.1451. World J Gastrointest Oncol. 2023. PMID: 37663936 Free PMC article.
-
Severe Anemia from Multiple Gastric Hyperplastic Polyps in a Hemodialysis Patient after Long-term Use of a Proton-pump Inhibitor.Intern Med. 2024;63(5):649-657. doi: 10.2169/internalmedicine.2091-23. Intern Med. 2024. PMID: 38432892 Free PMC article.
-
Gastric cancer in patients with Helicobacter pylori-negative autoimmune gastritis.World J Gastrointest Oncol. 2025 Apr 15;17(4):101661. doi: 10.4251/wjgo.v17.i4.101661. World J Gastrointest Oncol. 2025. PMID: 40235879 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

