Programmable Attenuation of Antigenic Sensitivity for a Nanobody-Based EGFR Chimeric Antigen Receptor Through Hinge Domain Truncation
- PMID: 35935988
- PMCID: PMC9354126
- DOI: 10.3389/fimmu.2022.864868
Programmable Attenuation of Antigenic Sensitivity for a Nanobody-Based EGFR Chimeric Antigen Receptor Through Hinge Domain Truncation
Abstract
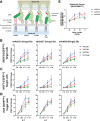
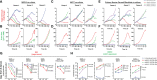
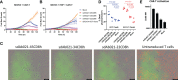
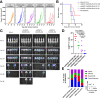
Epidermal growth factor family receptor (EGFR) is commonly overexpressed in many solid tumors and an attractive target for chimeric antigen receptor (CAR)-T therapy, but as EGFR is also expressed at lower levels in healthy tissues a therapeutic strategy must balance antigenic responsiveness against the risk of on-target off-tumor toxicity. Herein, we identify several camelid single-domain antibodies (also known as nanobodies) that are effective EGFR targeting moieties for CARs (EGFR-sdCARs) with very strong reactivity to EGFR-high and EGFR-low target cells. As a strategy to attenuate their potent antigenic sensitivity, we performed progressive truncation of the human CD8 hinge commonly used as a spacer domain in many CAR constructs. Single amino acid hinge-domain truncation progressively decreased both EGFR-sdCAR-Jurkat cell binding to EGFR-expressing targets and expression of the CD69 activation marker. Attenuated signaling in hinge-truncated EGFR-sdCAR constructs increased selectivity for antigen-dense EGFR-overexpressing cells over an EGFR-low tumor cell line or healthy donor derived EGFR-positive fibroblasts. We also provide evidence that epitope location is critical for determining hinge-domain requirement for CARs, as hinge truncation similarly decreased antigenic sensitivity of a membrane-proximal epitope targeting HER2-CAR but not a membrane-distal EGFRvIII-specific CAR. Hinge-modified EGFR-sdCAR cells showed clear functional attenuation in Jurkat-CAR-T cells and primary human CAR-T cells from multiple donors in vitro and in vivo. Overall, these results indicate that hinge length tuning provides a programmable strategy for throttling antigenic sensitivity in CARs targeting membrane-proximal epitopes, and could be employed for CAR-optimization and improved tumor selectivity.
Keywords: CAR optimization; CAR-T; EGFR; cancer selectivity; cell therapy; cellular immunotherapy; hinge domain.
Copyright © 2022 McComb, Nguyen, Shepherd, Henry, Bloemberg, Marcil, Maclean, Zafer, Gilbert, Gadoury, Pon, Sulea, Zhu and Weeratna.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Engineered Jurkat Cells for Targeting Prostate-Specific Membrane Antigen on Prostate Cancer Cells by Nanobody-Based Chimeric Antigen Receptor.Iran Biomed J. 2020 Mar;24(2):81-8. doi: 10.29252/ibj.24.2.81. Epub 2019 Oct 23. Iran Biomed J. 2020. PMID: 31677604 Free PMC article.
-
A Novel Siglec-4 Derived Spacer Improves the Functionality of CAR T Cells Against Membrane-Proximal Epitopes.Front Immunol. 2020 Aug 7;11:1704. doi: 10.3389/fimmu.2020.01704. eCollection 2020. Front Immunol. 2020. PMID: 32849600 Free PMC article.
-
Selective Targeting of Glioblastoma with EGFRvIII/EGFR Bitargeted Chimeric Antigen Receptor T Cell.Cancer Immunol Res. 2018 Nov;6(11):1314-1326. doi: 10.1158/2326-6066.CIR-18-0044. Epub 2018 Sep 10. Cancer Immunol Res. 2018. PMID: 30201736
-
The Application of Nanobody in CAR-T Therapy.Biomolecules. 2021 Feb 8;11(2):238. doi: 10.3390/biom11020238. Biomolecules. 2021. PMID: 33567640 Free PMC article. Review.
-
CAR T-cell bioengineering: Single variable domain of heavy chain antibody targeted CARs.Adv Drug Deliv Rev. 2019 Feb 15;141:41-46. doi: 10.1016/j.addr.2019.04.006. Epub 2019 Apr 17. Adv Drug Deliv Rev. 2019. PMID: 31004624 Review.
Cited by
-
A Flow Cytometry-Based Method for Assessing CAR Cell Binding Kinetics Using Stable CAR Jurkat Cells.Bio Protoc. 2024 Jun 20;14(12):e5021. doi: 10.21769/BioProtoc.5021. eCollection 2024 Jun 20. Bio Protoc. 2024. PMID: 38948258 Free PMC article.
-
Isolation and Characterization of Single-Domain Antibodies from Immune Phage Display Libraries.Methods Mol Biol. 2023;2702:107-147. doi: 10.1007/978-1-0716-3381-6_7. Methods Mol Biol. 2023. PMID: 37679618
-
Bispecific T-Cell Engagers and Chimeric Antigen Receptor T-Cell Therapies in Glioblastoma: An Update.Curr Oncol. 2023 Sep 15;30(9):8501-8549. doi: 10.3390/curroncol30090619. Curr Oncol. 2023. PMID: 37754534 Free PMC article. Review.
-
Entinostat, a histone deacetylase inhibitor, enhances CAR-NK cell anti-tumor activity by sustaining CAR expression.Front Immunol. 2025 Mar 7;16:1533044. doi: 10.3389/fimmu.2025.1533044. eCollection 2025. Front Immunol. 2025. PMID: 40124378 Free PMC article.
-
Delivery of Plasmid DNA by Ionizable Lipid Nanoparticles to Induce CAR Expression in T Cells.Int J Nanomedicine. 2023 Oct 18;18:5891-5904. doi: 10.2147/IJN.S424723. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37873551 Free PMC article.
References
-
- Wong WY, Tanha J, Krishnan L, Tian B, Kumar P, Gaspar K, et al. . Abstract A74: CAR-T Cells Harboring Camelid Single Domain Antibody as Targeting Agent to CEACAM6 Antigen in Pancreatic Cancer. Cancer Immunol Res (2017) 5:A74–4. doi: 10.1158/2326-6074.TUMIMM16-A74 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

