CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments
- PMID: 35936003
- PMCID: PMC9355792
- DOI: 10.3389/fimmu.2022.925985
CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments
Abstract
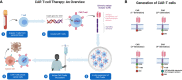
Chimeric antigen receptor (CAR)-T cell therapy is a progressive new pillar in immune cell therapy for cancer. It has yielded remarkable clinical responses in patients with B-cell leukemia or lymphoma. Unfortunately, many challenges remain to be addressed to overcome its ineffectiveness in the treatment of other hematological and solidtumor malignancies. The major hurdles of CAR T-cell therapy are the associated severe life-threatening toxicities such as cytokine release syndrome and limited anti-tumor efficacy. In this review, we briefly discuss cancer immunotherapy and the genetic engineering of T cells and, In detail, the current innovations in CAR T-cell strategies to improve efficacy in treating solid tumors and hematologic malignancies. Furthermore, we also discuss the current challenges in CAR T-cell therapy and new CAR T-cell-derived nanovesicle therapy. Finally, strategies to overcome the current clinical challenges associated with CAR T-cell therapy are included as well.
Keywords: CAR T-cell therapy; gene therapy; hematologic malignancies; immunotherapy; solid cancers.
Copyright © 2022 Jogalekar, Rajendran, Khan, Dmello, Gangadaran and Ahn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

