Hemidesmosomal Reactivity and Treatment Recommendations in Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid-A Retrospective, Monocentric Study
- PMID: 35936009
- PMCID: PMC9355658
- DOI: 10.3389/fimmu.2022.953546
Hemidesmosomal Reactivity and Treatment Recommendations in Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid-A Retrospective, Monocentric Study
Abstract
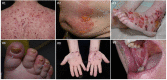
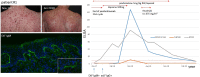
Immune checkpoint inhibitors (ICI) induce T-cell-mediated antitumour responses. While ICI were initially successfully applied in metastasized melanoma, they are now approved for several tumour entities. Numerous autoimmune disorders have been reported to occur as adverse events of the treatment, among them bullous pemphigoid (BP), with less than 1% of the patients experiencing ICI-induced BP. This number is higher than the estimated prevalence of autoimmune bullous diseases in the general population of Germany, which lies around 0.05%. We here describe our cohort of eight patients, who developed a bullous pemphigoid under or shortly after ICI treatment. Half of them had a severe subtype (as shown by BPDAI >57) and showed a median onset of ICI-BP after 10 months of ICI initiation. Six patients had a palmar and/or plantar involvement, while oral involvement occurred in one case. All patients had linear epidermal IgG depositions in split skin in the indirect immunofluorescence. In four out of five biopsies available for direct immunofluorescence, linear IgG and C3 depositions were detected at the basement membrane, while one patient showed linear IgM staining. Moderate to high levels of FLBP180 autoantibodies were found in seven of eight cases. The disease can still be active after ICI discontinuation, while rituximab might be required for remission. Finally, four tumour samples were stained histochemically for collagen XVII (BP180), but no enhanced expression was found.
Keywords: autoimmune bullous disorders; collagen XVII; immunosuppression; melanoma; skin fragility.
Copyright © 2022 Schauer, Rafei-Shamsabadi, Mai, Mai, Izumi, Meiss and Kiritsi.
Conflict of interest statement
FM served as a consultant and/or has received honoraria from Novartis, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Pierre Fabre, and Sanofi Genzyme and travel support from Novartis, Sunpharma and Bristol-Myers Squibb, outside the submitted work. DK served as a consultant and/or has received honoraria from Amryt Pharma, UCB, Novartis, Fibrx Derm and Colzyx. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


Similar articles
-
Basement membrane zone IgE deposition is associated with bullous pemphigoid disease severity and treatment results.Br J Dermatol. 2020 May;182(5):1221-1227. doi: 10.1111/bjd.18364. Epub 2019 Oct 16. Br J Dermatol. 2020. PMID: 31330562
-
Checkpoint Inhibition May Trigger the Rare Variant of Anti-LAD-1 IgG-Positive, Anti-BP180 NC16A IgG-Negative Bullous Pemphigoid.Front Immunol. 2019 Aug 14;10:1934. doi: 10.3389/fimmu.2019.01934. eCollection 2019. Front Immunol. 2019. PMID: 31474998 Free PMC article.
-
Checkpoint inhibitor-associated bullous cutaneous immune-related adverse events: a multicentre observational study.Br J Dermatol. 2022 Dec;187(6):981-987. doi: 10.1111/bjd.21836. Epub 2022 Sep 6. Br J Dermatol. 2022. PMID: 35976170
-
Case Report: Bullous Pemphigoid Associated With Morphea and Lichen Sclerosus: Coincidental Diseases or Pathogenetic Association?Front Immunol. 2022 May 3;13:887279. doi: 10.3389/fimmu.2022.887279. eCollection 2022. Front Immunol. 2022. PMID: 35592319 Free PMC article. Review.
-
Bullous pemphigoid (BP) patients with selective IgG autoreactivity against BP230: Review of a rare but valuable cohort with impact on the comprehension of the pathogenesis of BP.J Dermatol Sci. 2022 Feb;105(2):72-79. doi: 10.1016/j.jdermsci.2021.11.011. Epub 2021 Dec 1. J Dermatol Sci. 2022. PMID: 34930674 Review.
Cited by
-
The Use of Biologic Agents for the Treatment of Cutaneous Immune-Related Adverse Events from Immune Checkpoint Inhibitors: A Review of Reported Cases.Am J Clin Dermatol. 2024 Jul;25(4):595-607. doi: 10.1007/s40257-024-00866-z. Epub 2024 May 20. Am J Clin Dermatol. 2024. PMID: 38767827 Review.
-
A Review of Bullous Dermatologic Adverse Events Associated with Anti-Cancer Therapy.Biomedicines. 2023 Jan 24;11(2):323. doi: 10.3390/biomedicines11020323. Biomedicines. 2023. PMID: 36830860 Free PMC article. Review.
-
Talimogene laherparepvec induced bullous pemphigoid.JAAD Case Rep. 2025 Apr 2;60:115-117. doi: 10.1016/j.jdcr.2025.03.016. eCollection 2025 Jun. JAAD Case Rep. 2025. PMID: 40417130 Free PMC article. No abstract available.
-
Successful Treatment of Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid with Omalizumab: A Case Report and Review of the Literature.Clin Cosmet Investig Dermatol. 2024 Dec 14;17:2865-2874. doi: 10.2147/CCID.S487711. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39697463 Free PMC article.
-
The possible and intriguing relationship between bullous pemphigoid and melanoma: speculations on significance and clinical relevance.Front Immunol. 2024 Aug 29;15:1416473. doi: 10.3389/fimmu.2024.1416473. eCollection 2024. Front Immunol. 2024. PMID: 39267741 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

