Hemidesmosomal Reactivity and Treatment Recommendations in Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid-A Retrospective, Monocentric Study
- PMID: 35936009
- PMCID: PMC9355658
- DOI: 10.3389/fimmu.2022.953546
Hemidesmosomal Reactivity and Treatment Recommendations in Immune Checkpoint Inhibitor-Induced Bullous Pemphigoid-A Retrospective, Monocentric Study
Abstract
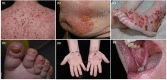
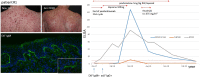
Immune checkpoint inhibitors (ICI) induce T-cell-mediated antitumour responses. While ICI were initially successfully applied in metastasized melanoma, they are now approved for several tumour entities. Numerous autoimmune disorders have been reported to occur as adverse events of the treatment, among them bullous pemphigoid (BP), with less than 1% of the patients experiencing ICI-induced BP. This number is higher than the estimated prevalence of autoimmune bullous diseases in the general population of Germany, which lies around 0.05%. We here describe our cohort of eight patients, who developed a bullous pemphigoid under or shortly after ICI treatment. Half of them had a severe subtype (as shown by BPDAI >57) and showed a median onset of ICI-BP after 10 months of ICI initiation. Six patients had a palmar and/or plantar involvement, while oral involvement occurred in one case. All patients had linear epidermal IgG depositions in split skin in the indirect immunofluorescence. In four out of five biopsies available for direct immunofluorescence, linear IgG and C3 depositions were detected at the basement membrane, while one patient showed linear IgM staining. Moderate to high levels of FLBP180 autoantibodies were found in seven of eight cases. The disease can still be active after ICI discontinuation, while rituximab might be required for remission. Finally, four tumour samples were stained histochemically for collagen XVII (BP180), but no enhanced expression was found.
Keywords: autoimmune bullous disorders; collagen XVII; immunosuppression; melanoma; skin fragility.
Copyright © 2022 Schauer, Rafei-Shamsabadi, Mai, Mai, Izumi, Meiss and Kiritsi.
Conflict of interest statement
FM served as a consultant and/or has received honoraria from Novartis, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Pierre Fabre, and Sanofi Genzyme and travel support from Novartis, Sunpharma and Bristol-Myers Squibb, outside the submitted work. DK served as a consultant and/or has received honoraria from Amryt Pharma, UCB, Novartis, Fibrx Derm and Colzyx. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

