Low-dose mycophenolate mofetil improves survival in a murine model of Staphylococcus aureus sepsis by increasing bacterial clearance and phagocyte function
- PMID: 35936013
- PMCID: PMC9351454
- DOI: 10.3389/fimmu.2022.939213
Low-dose mycophenolate mofetil improves survival in a murine model of Staphylococcus aureus sepsis by increasing bacterial clearance and phagocyte function
Abstract
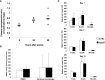
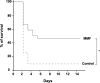
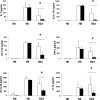
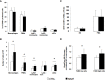
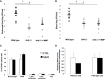
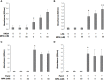
Regulators of TLRs signaling pathways play an important role in the control of the pro-inflammatory response that contributes to sepsis-induced tissue injury. Mycophenolate mofetil, an immunosuppressive drug inhibiting lymphocyte proliferation, has been reported to be a regulator of TLRs signaling pathways. Whether MMF used at infra-immunosuppressive doses has an impact on survival and on innate immune response in sepsis is unknown. C57BL/6J mice were infected intraperitoneally with 108 CFU Staphylococcus aureus, and treated or not with low-dose of MMF (20mg/kg/day during 4 days). Survival rate and bacterial clearance were compared. Cytokine levels, quantitative and qualitative cellular responses were assessed. S. aureus - infected mice treated with MMF exhibited improved survival compared to non-treated ones (48% vs 10%, p<0.001). With the dose used for all experiments, MMF did not show any effect on lymphocyte proliferation. MMF treatment also improved local and systemic bacterial clearance, improved phagocytosis activity of peritoneal macrophages resulting in decreased inflammatory cytokines secretion. MMF-treated mice showed enhanced activation of NF-κB seemed with a suspected TLR4-dependent mechanism. These results suggest that infra-immunosuppressive doses of MMF improve host defense during S. aureus sepsis and protects infected mice from fatal outcome by regulating innate immune responses. The signaling pathways involved could be TLR4-dependent. This work brings new perspectives in pathogenesis and therapeutic approaches of severe infections.
Keywords: NF-κB; innate immunity; macrophages; mycophenolate mofetil; phagocytosis; sepsis; staphylococcus aureus; toll-like receptor 4.
Copyright © 2022 Alby-Laurent, Belaïdouni, Blanchet, Rousseau, Llitjos, Sanquer, Mira, Pène, Toubiana and Chiche.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Mycophenolate mofetil attenuates concanavalin A-induced acute liver injury through modulation of TLR4/NF-κB and Nrf2/HO-1 pathways.Pharmacol Rep. 2020 Aug;72(4):945-955. doi: 10.1007/s43440-019-00055-4. Epub 2020 Jan 14. Pharmacol Rep. 2020. PMID: 32048261
-
Mycophenolate Mofetil Protects Septic Mice via the Dual Inhibition of Inflammatory Cytokines and PD-1.Inflammation. 2018 Jun;41(3):1008-1020. doi: 10.1007/s10753-018-0754-2. Inflammation. 2018. PMID: 29455288
-
Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic T-lymphocyte and interleukin-17-mediated macrophage accumulation.J Am Coll Cardiol. 2011 May 24;57(21):2194-204. doi: 10.1016/j.jacc.2010.12.030. J Am Coll Cardiol. 2011. PMID: 21596236 Free PMC article.
-
Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF).Clin Transplant. 1996 Feb;10(1 Pt 2):77-84. Clin Transplant. 1996. PMID: 8680053 Review.
-
Evaluation of effect of empirical attack-preventive immunotherapies in neuromyelitis optica spectrum disorders: An update systematic review and meta -analysis.J Neuroimmunol. 2022 Feb 15;363:577790. doi: 10.1016/j.jneuroim.2021.577790. Epub 2021 Dec 16. J Neuroimmunol. 2022. PMID: 34959021
Cited by
-
Nrf2/PHB2 alleviates mitochondrial damage and protects against Staphylococcus aureus-induced acute lung injury.MedComm (2020). 2023 Dec 7;4(6):e448. doi: 10.1002/mco2.448. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38077250 Free PMC article.
-
Mycophenolate mofetil directly modulates myeloid viability and pro-fibrotic activation of human macrophages.Rheumatology (Oxford). 2025 May 1;64(5):3125-3133. doi: 10.1093/rheumatology/keae517. Rheumatology (Oxford). 2025. PMID: 39312626
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

