Palladium-catalyzed allylic etherification of phenols with vinyl ethylene carbonate
- PMID: 35936101
- PMCID: PMC9354801
- DOI: 10.3389/fchem.2022.962355
Palladium-catalyzed allylic etherification of phenols with vinyl ethylene carbonate
Abstract
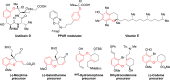
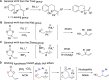
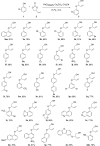
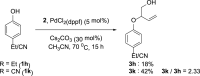
The palladium-catalyzed decarboxylative reactions of phenols and vinyl ethylene carbonate to produce allylic aryl ethers under mild conditions have been established. Adopting an inexpensive PdCl2(dppf) catalyst promotes the efficient conversion of phenols to the corresponding allylic aryl ethers via the formation of a new C-O bond in good isolated yields with complete regioselectivities, acceptable functional group tolerance and operational simplicity. The robust procedure could be completed smoothly by conducting a scaled-up reaction with comparable efficiency to afford the target product.
Keywords: C-O bond formation; allylic etherification; palladium-catalyzed; phenols; vinyl ethylene carbonate.
Copyright © 2022 Lin, Zhao, He, Li, Jiang, Xiang, Ye and Gan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Consiglio G., Waymouth R. M. (1989). Enantioselective homogeneous catalysis involving transition-metal-allyl intermediates. Chem. Rev. 89, 257–276. 10.1021/cr00091a007 - DOI
LinkOut - more resources
Full Text Sources

