Granzyme B PET Imaging Stratifies Immune Checkpoint Inhibitor Response in Hepatocellular Carcinoma
- PMID: 35936114
- PMCID: PMC9328186
- DOI: 10.1155/2021/9305277
Granzyme B PET Imaging Stratifies Immune Checkpoint Inhibitor Response in Hepatocellular Carcinoma
Abstract
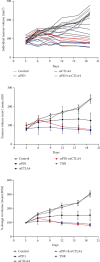
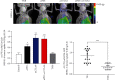
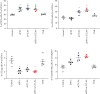
Hepatocellular carcinoma (HCC) is a notoriously difficult cancer to treat. The recent development of immune checkpoint inhibitors has revolutionised HCC therapy; however, successful response is only observed in a small percentage of patients. Biomarkers typically used to predict treatment response in other tumour types are ineffective in HCC, which arises in an immune-suppressive environment. However, imaging markers that measure changes in tumour infiltrating immune cells may supply information that can be used to determine which patients are responding to therapy posttreatment. We have evaluated [18F]AlF-mNOTA-GZP, a radiolabeled peptide targeting granzyme B, to stratify response to ICIs in a HEPA 1-tumours, a syngeneic model of HCC. Posttherapy, in vivo tumour retention of [18F]AlF-mNOTA-GZP was correlated to changes in tumour volume and tumour-infiltrating immune cells. [18F]AlF-mNOTA-GZP successfully stratified response to immune checkpoint inhibition in the syngeneic HEPA 1-6 model. FACS indicated significant changes in the immune environment including a decrease in immune suppressive CD4+ T regulatory cells and increases in tumour-associated GZB+ NK+ cells, which correlated well with tumour radiopharmaceutical uptake. While the immune response to ICI therapies differs in HCC compared to many other cancers, [18F]AlF-mNOTA-GZP retention is able to stratify response to ICI therapy associated with tumour infiltrating GZB+ NK+ cells in this complex tumour microenvironment.
Copyright © 2021 Julian L. Goggi et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Granzyme B PET Imaging of Combined Chemotherapy and Immune Checkpoint Inhibitor Therapy in Colon Cancer.Mol Imaging Biol. 2021 Oct;23(5):714-723. doi: 10.1007/s11307-021-01596-y. Epub 2021 Mar 12. Mol Imaging Biol. 2021. PMID: 33713000 Free PMC article.
-
Granzyme B PET Imaging of Immune Checkpoint Inhibitor Combinations in Colon Cancer Phenotypes.Mol Imaging Biol. 2020 Oct;22(5):1392-1402. doi: 10.1007/s11307-020-01519-3. Mol Imaging Biol. 2020. PMID: 32705455 Free PMC article.
-
Granzyme B PET Imaging in Response to In Situ Vaccine Therapy Combined with αPD1 in a Murine Colon Cancer Model.Pharmaceutics. 2022 Jan 8;14(1):150. doi: 10.3390/pharmaceutics14010150. Pharmaceutics. 2022. PMID: 35057046 Free PMC article.
-
Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma.J Immunother Cancer. 2020 Apr;8(1):e000394. doi: 10.1136/jitc-2019-000394. J Immunother Cancer. 2020. PMID: 32303615 Free PMC article.
-
The association between tumour heterogeneity and immune evasion mechanisms in hepatocellular carcinoma and its clinical implications.Br J Cancer. 2024 Aug;131(3):420-429. doi: 10.1038/s41416-024-02684-w. Epub 2024 May 17. Br J Cancer. 2024. PMID: 38760445 Free PMC article. Review.
Cited by
-
Time to consider sequencing anti-inflammatory treatments with chemotherapy and immuno-stimulation?Front Immunol. 2022 Dec 22;13:1082142. doi: 10.3389/fimmu.2022.1082142. eCollection 2022. Front Immunol. 2022. PMID: 36618422 Free PMC article. No abstract available.
-
Illuminating immunotherapy response via precision T cell-targeted PET imaging.Front Med (Lausanne). 2024 Jul 22;11:1233913. doi: 10.3389/fmed.2024.1233913. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39104861 Free PMC article. Review.
References
-
- Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. The Lancet Oncology . 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

