Pembrolizumab-Induced Pancreatic Exocrine Insufficiency Complicated by Severe Hepatic Steatosis
- PMID: 35936135
- PMCID: PMC9354921
- DOI: 10.7759/cureus.26596
Pembrolizumab-Induced Pancreatic Exocrine Insufficiency Complicated by Severe Hepatic Steatosis
Abstract
Anti-programmed death receptor-1 (anti-PD-1) monoclonal antibodies (mAbs) are used to treat an increasing range of cancers. However, the distinct toxicity profile of immune-related adverse events (irAEs) is a frequent drawback of their clinical application. Among the more common irAEs are hepatitis and colitis, which are diagnosed and graded in patients based on elevated serum liver enzyme levels and increased stool frequency, respectively, and both of which often require treatment with high-dose corticosteroids. Herein, we describe the case of a patient who developed severe transaminase elevation and diarrhoea due to an unusual irAE, which was successfully treated without corticosteroids.
Keywords: hepatic steatosis; immune-related adverse event; immunotherapy; pancreatic exocrine insufficiency; pembrolizumab.
Copyright © 2022, Hong et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
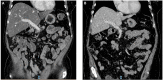

Similar articles
-
Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors.J Hepatol. 2018 Jun;68(6):1181-1190. doi: 10.1016/j.jhep.2018.01.033. Epub 2018 Feb 8. J Hepatol. 2018. PMID: 29427729
-
Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy.Immunotherapy. 2018 Mar;10(3):171-175. doi: 10.2217/imt-2017-0126. Immunotherapy. 2018. PMID: 29370723
-
Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe gastrointestinal and hepatic toxicities from checkpoint inhibitors.Support Care Cancer. 2020 Dec;28(12):6129-6143. doi: 10.1007/s00520-020-05707-3. Epub 2020 Aug 27. Support Care Cancer. 2020. PMID: 32856210 Free PMC article. Review.
-
Case report: reinitiating pembrolizumab treatment after small bowel perforation.BMC Cancer. 2019 Apr 24;19(1):379. doi: 10.1186/s12885-019-5577-5. BMC Cancer. 2019. PMID: 31018834 Free PMC article.
-
Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents.Oncologist. 2016 Oct;21(10):1230-1240. doi: 10.1634/theoncologist.2016-0055. Epub 2016 Jul 8. Oncologist. 2016. PMID: 27401894 Free PMC article. Review.
Cited by
-
Immune checkpoint inhibitor associated diarrhoea.BMJ Case Rep. 2024 May 8;17(5):e259057. doi: 10.1136/bcr-2023-259057. BMJ Case Rep. 2024. PMID: 38719255
-
Etiologies of exocrine pancreatic insufficiency.Gastroenterol Rep (Oxf). 2025 Mar 10;13:goaf019. doi: 10.1093/gastro/goaf019. eCollection 2025. Gastroenterol Rep (Oxf). 2025. PMID: 40066317 Free PMC article. Review.
-
Different activity and toxicity of immunotherapy in monozygotic twins diagnosed with early triple-negative breast cancer: a case report.Ther Adv Med Oncol. 2025 Feb 18;17:17588359241297565. doi: 10.1177/17588359241297565. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 39975510 Free PMC article.
-
Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy.Metabolites. 2025 Jun 10;15(6):385. doi: 10.3390/metabo15060385. Metabolites. 2025. PMID: 40559409 Free PMC article. Review.
References
-
- Autoimmune pancreatitis after nivolumab anti-programmed death receptor-1 treatment. Dehghani L, Mikail N, Kramkimel N, Soyer P, Lebtahi R, Mallone R, Larger E. Eur J Cancer. 2018;104:243–246. - PubMed
-
- Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Hofmann L, Forschner A, Loquai C, et al. Eur J Cancer. 2016;60:190–209. - PubMed
-
- Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Ann Oncol. 2017;28:119–142. - PubMed
-
- Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. De Martin E, Michot JM, Papouin B, et al. J Hepatol. 2018;68:1181–1190. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
