Hierarchical Na3V2(PO4)2F3 Microsphere Cathodes for High-Temperature Li-Ion Battery Application
- PMID: 35936407
- PMCID: PMC9352260
- DOI: 10.1021/acsomega.2c02558
Hierarchical Na3V2(PO4)2F3 Microsphere Cathodes for High-Temperature Li-Ion Battery Application
Abstract
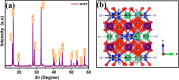
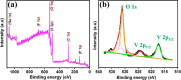
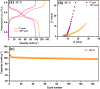
Sodium superionic conductor (NASICON)-structured Na3V2(PO4)2F3 cathode materials have received vast attention in the high-temperature storage performance due to their structural and thermal stability. Herein, hierarchical Na3V2(PO4)2F3 microspheres (NVPF-HMSs) consisting of nanocubes were designed by a one-pot facial solvothermal method. The hierarchical Na3V2(PO4)2F3 microsphere size is 2-3 μm, which is corroborated by FE-SEM and HR-TEM analyses. The NVPF-HMSs have been demonstrated as a cathode in Li-ion batteries at both low and elevated temperatures (25 and 55 °C, respectively). The NVPF-HMS cathode in a Li-ion cell exhibits reversible capacities of 119 mA h g-1 at 0.1 C and 85 mA h g-1 at 1 C with an 82% retention after 250 cycles at 25 °C. At elevated temperatures, the NVPF-HMS cathode exhibits a superior capacity of 110 mA h g-1 at 1 C along with a retention of 90% after 150 cycles at 55 °C. Excellent capacity and cyclability were achieved at 55 °C due to its hierarchical morphology with a robust crystal structure, low charge-transfer resistance, and improved ionic diffusivity. The Li-ion storage performance of the NVPF-HMS cathode material at elevated temperatures was analyzed for the first time to understand the high-temperature storage property of the material, and it was found to be a promising candidate for elevated-temperature energy storage applications.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Yoo H. D.; Markevich E.; Salitra G.; Sharon D.; Aurbach D. On the Challenge of Developing Advanced Technologies for Electrochemical Energy Storage and Conversion. Mater. Today 2014, 17, 110–121. 10.1016/j.mattod.2014.02.014. - DOI
-
- Goodenough J. B.; Kim Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. 10.1021/cm901452z. - DOI
LinkOut - more resources
Full Text Sources

