Contributions of Astrocyte and Neuronal Volume to CA1 Neuron Excitability Changes in Elevated Extracellular Potassium
- PMID: 35936495
- PMCID: PMC9352931
- DOI: 10.3389/fncel.2022.930384
Contributions of Astrocyte and Neuronal Volume to CA1 Neuron Excitability Changes in Elevated Extracellular Potassium
Abstract
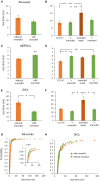
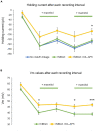
Rapid increases in cell volume reduce the size of the extracellular space (ECS) and are associated with elevated brain tissue excitability. We recently demonstrated that astrocytes, but not neurons, rapidly swell in elevated extracellular potassium (∧[K+] o ) up to 26 mM. However, effects of acute astrocyte volume fluctuations on neuronal excitability in ∧[K+] o have been difficult to evaluate due to direct effects on neuronal membrane potential and generation of action potentials. Here we set out to isolate volume-specific effects occurring in ∧[K+] o on CA1 pyramidal neurons in acute hippocampal slices by manipulating cell volume while recording neuronal glutamate currents in 10.5 mM [K+] o + tetrodotoxin (TTX) to prevent neuronal firing. Elevating [K+] o to 10.5 mM induced astrocyte swelling and produced significant increases in neuronal excitability in the form of mixed α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/N-methyl-D-aspartate (NMDA) receptor mEPSCs and NMDA receptor-dependent slow inward currents (SICs). Application of hyperosmolar artificial cerebrospinal fluid (ACSF) by addition of mannitol in the continued presence of 10.5 mM K+ forced shrinking of astrocytes and to a lesser extent neurons, which resisted swelling in ∧[K+] o . Cell shrinking and dilation of the ECS significantly dampened neuronal excitability in 10.5 mM K+. Subsequent removal of mannitol amplified effects on neuronal excitability and nearly doubled the volume increase in astrocytes, presumably due to continued glial uptake of K+ while mannitol was present. Slower, larger amplitude events mainly driven by NMDA receptors were abolished by mannitol-induced expansion of the ECS. Collectively, our findings suggest that cell volume regulation of the ECS in elevated [K+] o is driven predominantly by astrocytes, and that cell volume effects on neuronal excitability can be effectively isolated in elevated [K+] o conditions.
Keywords: NMDA; cell swelling; epilepsy; extracellular space; extrasynaptic; glutamate; secretagogue; slow inward currents.
Copyright © 2022 Walch, Bilas, Bebawy, Lam, Murphy, Sriram and Fiacco.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Agnati L. F., Zoli M., Strömberg I., Fuxe K. (1995). Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69 711–726. - PubMed
-
- Bach-y-Rita P. (1993). Neurotransmission in the brain by diffusion through the extracellular fluid: a review. Neuroreport 4 343–350. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

