Mechanistic Insights into Enzyme Catalysis from Explaining Machine-Learned Quantum Mechanical and Molecular Mechanical Minimum Energy Pathways
- PMID: 35936506
- PMCID: PMC9344433
- DOI: 10.1021/acsphyschemau.2c00005
Mechanistic Insights into Enzyme Catalysis from Explaining Machine-Learned Quantum Mechanical and Molecular Mechanical Minimum Energy Pathways
Abstract
With the increasing popularity of machine learning (ML) applications, the demand for explainable artificial intelligence techniques to explain ML models developed for computational chemistry has also emerged. In this study, we present the development of the Boltzmann-weighted cumulative integrated gradients (BCIG) approach for effective explanation of mechanistic insights into ML models trained on high-level quantum mechanical and molecular mechanical (QM/MM) minimum energy pathways. Using the acylation reactions of the Toho-1 β-lactamase and two antibiotics (ampicillin and cefalexin) as the model systems, we show that the BCIG approach could quantitatively attribute the energetic contribution in one system and the relative reactivity of individual steps across different systems to specific chemical processes such as the bond making/breaking and proton transfers. The proposed BCIG contribution attribution method quantifies chemistry-interpretable insights in terms of contributions from each elementary chemical process, which is in agreement with the validating QM/MM calculations and our intuitive mechanistic understandings of the model reactions.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


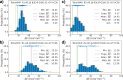
 ”, “mean
”, “mean  ”, “med. ΔE”, and “std”
refer to the minimum, exponential
average, arithmetic average, median, and standard deviation of the
energy barriers from the QM/MM MEP profiles, respectively.
”, “med. ΔE”, and “std”
refer to the minimum, exponential
average, arithmetic average, median, and standard deviation of the
energy barriers from the QM/MM MEP profiles, respectively.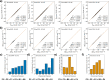


Similar articles
-
Unraveling the energetic significance of chemical events in enzyme catalysis via machine-learning based regression approach.Commun Chem. 2020 Oct 8;3(1):134. doi: 10.1038/s42004-020-00379-w. Commun Chem. 2020. PMID: 36703376 Free PMC article.
-
Reaction path potential for complex systems derived from combined ab initio quantum mechanical and molecular mechanical calculations.J Chem Phys. 2004 Jul 1;121(1):89-100. doi: 10.1063/1.1757436. J Chem Phys. 2004. PMID: 15260525
-
Free Energy Contribution Analysis Using Response Kernel Approximation: Insights into the Acylation Reaction of a Beta-Lactamase.J Phys Chem B. 2016 Sep 8;120(35):9338-46. doi: 10.1021/acs.jpcb.6b06104. Epub 2016 Aug 25. J Phys Chem B. 2016. PMID: 27501066
-
Modeling biotransformation reactions by combined quantum mechanical/molecular mechanical approaches: from structure to activity.Curr Top Med Chem. 2003;3(11):1241-56. doi: 10.2174/1568026033452005. Curr Top Med Chem. 2003. PMID: 12769703 Review.
-
Organic reactivity from mechanism to machine learning.Nat Rev Chem. 2021 Apr;5(4):240-255. doi: 10.1038/s41570-021-00260-x. Epub 2021 Mar 16. Nat Rev Chem. 2021. PMID: 37117288 Review.
Cited by
-
Prediction of Enzyme Catalysis by Computing Reaction Energy Barriers via Steered QM/MM Molecular Dynamics Simulations and Machine Learning.J Chem Inf Model. 2023 Aug 14;63(15):4623-4632. doi: 10.1021/acs.jcim.3c00772. Epub 2023 Jul 21. J Chem Inf Model. 2023. PMID: 37479222 Free PMC article.
-
Trends in in-silico guided engineering of efficient polyethylene terephthalate (PET) hydrolyzing enzymes to enable bio-recycling and upcycling of PET.Comput Struct Biotechnol J. 2023 Jun 5;21:3513-3521. doi: 10.1016/j.csbj.2023.06.004. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37484494 Free PMC article. Review.
-
Multiscale calculations reveal new insights into the reaction mechanism between KRASG12C and α, β-unsaturated carbonyl of covalent inhibitors.Comput Struct Biotechnol J. 2024 Apr 4;23:1408-1417. doi: 10.1016/j.csbj.2024.03.027. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38616962 Free PMC article.
-
Unveiling the structural features that regulate carbapenem deacylation in KPC-2 through QM/MM and interpretable machine learning.Phys Chem Chem Phys. 2023 Jan 4;25(2):1349-1362. doi: 10.1039/d2cp03724f. Phys Chem Chem Phys. 2023. PMID: 36537692 Free PMC article.
References
LinkOut - more resources
Full Text Sources
