Efficacy and Tolerability of Erenumab for Prevention of Episodic Migraine in India
- PMID: 35936611
- PMCID: PMC9350805
- DOI: 10.4103/aian.aian_199_22
Efficacy and Tolerability of Erenumab for Prevention of Episodic Migraine in India
Abstract
Background: EMPOwER, a 12-week, double-blind (DB), randomized, placebo-controlled study evaluated the efficacy and safety of erenumab in adult patients with episodic migraine (EM) from Asia, the Middle East, and Latin America. This study analyzes the Indian experience for the use of erunumab for prevention of episodic migraine.
Objective: The study aimed to evaluate the efficacy and tolerability of erenumab (70 mg and 140 mg) in EM patients from India.
Methods: Randomized patients received monthly subcutaneous injections of placebo and erenumab 70 mg or 140 mg for 3 months. The primary endpoint was a change from the baseline in monthly migraine days (MMDs) at month 3. Other endpoints included achievement of ≥50%, ≥75%, and 100% reduction in MMD; a change in monthly acute migraine-specific medication treatment days; a change in patient-reported outcomes; and safety assessment.
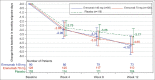
Results: Of the 539 patients screened, 351 patients were randomized (erenumab, 70 mg: n = 133 and 140 mg: n = 94; placebo: n = 124). The mean (±SD) age, disease duration, and MMD were 35.1 (±8.6) years, 6.77 (±6.01) years, and 7.82 (±2.89) days, respectively. The placebo-adjusted difference in mean MMD for erenumab 70 mg was -0.88 (95% CI, -2.16, 0.39; P = 0.174) days, and that for erenumab 140 mg was -1.01 (-2.42, 0.41; P = 0.164) days versus placebo. Secondary and exploratory endpoints demonstrated consistently better results in both erenumab dosage groups versus placebo. Treatment-emergent adverse events were comparable across groups (erenumab, 70 mg: 22.7% and 140 mg: 24.5%; placebo: 25.2%).
Conclusion: Both doses of erenumab showed numerical improvement for efficacy endpoints and were well-tolerated in the Indian population. No new safety signals were reported.
Keywords: Efficacy; Indian population; erenumab; headache; tolerability episodic migraine.
Copyright: © 2022 Annals of Indian Academy of Neurology.
Conflict of interest statement
Debashish Chowdhury, Jaydip Ray Chaudhuri, Pahari Ghosh, Rahul Kulkarni are the principal investigators for Novartis sponsored trials. Sumit Singh has attended advisory boards and is a speaker in Novartis sponsored meetings. Sneha Thakur and Anup Thorat are full time employees of Novartis, India.
Figures
References
-
- Ashina M. Migraine. N Engl J Med. 2020;3839:1866–76. - PubMed
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 19902016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. - PMC - PubMed
-
- Buse DC, Nicholson RA, Araujo AB, Reed ML, Shapiro RE, Ashina S, et al. Migraine care across the healthcare landscape in the United States among those with 4 or greater migraine headache days per month: Results of the OVERCOME study. Headache. 2019;59:16.
LinkOut - more resources
Full Text Sources
Miscellaneous