Immune Reconstitution of Patients Who Recovered From Steroid-Refractory Acute Graft-Versus-Host Disease After Basiliximab Treatment
- PMID: 35936697
- PMCID: PMC9351448
- DOI: 10.3389/fonc.2022.916442
Immune Reconstitution of Patients Who Recovered From Steroid-Refractory Acute Graft-Versus-Host Disease After Basiliximab Treatment
Abstract
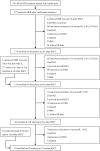
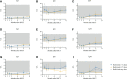
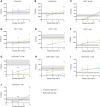
We aimed to identify the characteristics of immune reconstitution (IR) in patients who recovered from steroid-refractory acute graft-versus-host disease (SR-aGVHD) after basiliximab treatment. A total of 179, 124, 80, and 92 patients were included in the analysis for IR at 3, 6, 9, and 12 months, respectively, after haploidentical donor hematopoietic stem cell transplantation (HID HSCT). We observed that IR was fastest for monocytes and CD8+ T cells, followed by lymphocytes, CD3+ T cells, and CD19+ B cells and slowest for CD4+ T cells. Almost all immune cell subsets recovered comparably between patients receiving <5 doses and ≥5 doses of basiliximab. Most immune cell subsets recovered comparably between SR-aGVHD patients who recovered after basiliximab treatment and event-free HID HSCT recipients. Patients who recovered from SR-aGVHD after basiliximab treatment experienced satisfactory IR, which suggested that basiliximab may not have prolonged the negative impact on IR in these patients.
Keywords: Immune reconstitution; acute graft-versus-host disease; allogeneic hematopoietic stem cell transplantation; basiliximab; haploidentical; steroid-refractory.
Copyright © 2022 Deng, Fan, Zhang, Xu, Wang, Yan, Chen, Chen, Han, Wang, Wang, Pei, Chang, Liu, Huang and Mo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mesenchymal stromal cells plus basiliximab improve the response of steroid-refractory acute graft-versus-host disease as a second-line therapy: a multicentre, randomized, controlled trial.BMC Med. 2024 Feb 27;22(1):85. doi: 10.1186/s12916-024-03275-5. BMC Med. 2024. PMID: 38413930 Free PMC article. Clinical Trial.
-
Basiliximab as Treatment for Steroid-Refractory Acute Graft-versus-Host Disease in Pediatric Patients after Haploidentical Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2020 Feb;26(2):351-357. doi: 10.1016/j.bbmt.2019.10.031. Epub 2019 Nov 5. Biol Blood Marrow Transplant. 2020. PMID: 31704470
-
Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: Updated experience from a large-scale study.Am J Hematol. 2020 Aug;95(8):927-936. doi: 10.1002/ajh.25839. Epub 2020 May 7. Am J Hematol. 2020. PMID: 32311156
-
Meta-Analysis of Interleukin-2 Receptor Antagonists as the Treatment for Steroid-Refractory Acute Graft-Versus-Host Disease.Front Immunol. 2021 Sep 21;12:749266. doi: 10.3389/fimmu.2021.749266. eCollection 2021. Front Immunol. 2021. PMID: 34621279 Free PMC article.
-
Steroid-Refractory Gut Graft-Versus-Host Disease: What We Have Learned From Basic Immunology and Experimental Mouse Model.Front Immunol. 2022 Feb 18;13:844271. doi: 10.3389/fimmu.2022.844271. eCollection 2022. Front Immunol. 2022. PMID: 35251043 Free PMC article. Review.
Cited by
-
Time-dependent analysis of the impact on early cytomegalovirus reactivation of HLA mismatch and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation from related donors in acquired aplastic anemia.Ann Hematol. 2023 Sep;102(9):2589-2598. doi: 10.1007/s00277-023-05332-0. Epub 2023 Jul 13. Ann Hematol. 2023. PMID: 37438489
-
Lower dose of ATG combined with basiliximab for haploidentical hematopoietic stem cell transplantation is associated with effective control of GVHD and less CMV viremia.Front Immunol. 2022 Nov 15;13:1017850. doi: 10.3389/fimmu.2022.1017850. eCollection 2022. Front Immunol. 2022. PMID: 36458000 Free PMC article.
References
-
- Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. . The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2 - DOI - PMC - PubMed
-
- Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. . Controlled, Randomized, Open-Label Trial of Risk-Stratified Corticosteroid Prevention of Acute Graft-Versus-Host Disease After Haploidentical Transplantation. J Clin Oncol (2016) 34(16):1855–63. doi: 10.1200/JCO.2015.63.8817 - DOI - PubMed
-
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. . First- and Second-Line Systemic Treatment of Acute Graft-Versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

