Cost-Utility Analysis of Camrelizumab Plus Chemotherapy Versus Chemotherapy Alone as a First-Line Treatment for Advanced Nonsquamous Non-Small Cell Lung Cancer in China
- PMID: 35936702
- PMCID: PMC9353739
- DOI: 10.3389/fonc.2022.746526
Cost-Utility Analysis of Camrelizumab Plus Chemotherapy Versus Chemotherapy Alone as a First-Line Treatment for Advanced Nonsquamous Non-Small Cell Lung Cancer in China
Abstract
Purpose: To evaluate the cost utility of camrelizumab plus standard chemotherapy versus standard chemotherapy alone as a first-line treatment for advanced nonsquamous non-small cell lung cancer (NSCLC) from the perspective of the Chinese health care system and to provide a reference for health decision-making.
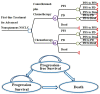
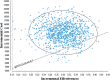
Methods: A Markov model consisting of three health states was designed to evaluate the cost utility of these two treatment regimens for NSCLC patients with the incremental cost-effectiveness ratio (ICER) as the primary output indicator. Clinical data were derived from a published phase III clinical trial (CameL; ClinicalTrials.gov; NCT03134872). One-way sensitivity analysis and probabilistic sensitivity analysis were performed to assess the model uncertainty.
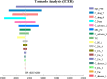
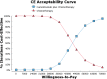
Results: Base case analysis showed that the ICER of camrelizumab plus chemotherapy compared with chemotherapy alone was $43,275.43 per QALY. It was higher than the willingness-to-pay (WTP) threshold of $31,510.57 per QALY in China, which has a standard of three times the GDP per capita recommended by the WHO. One-way sensitivity analysis showed that the utility value of PFS had the greatest influence on the results, and the other sensitive parameters were the cost of subsequent second-line therapy in the two group, the pemetrexed price, the cost of adverse event management and the utility value of PD. The probability sensitivity analysis showed that the probabilities of the cost-effectiveness of camrelizumab plus standard chemotherapy were 27.1%, 66.7% and 88.0% when the WTP values were $40,000, $50,000 and $60,000 per QALY, respectively.
Conclusions: Taking three times the GDP per capita in China as the WTP threshold, the camrelizumab plus standard chemotherapy regimen does not have a cost-effectiveness advantage compared with the standard chemotherapy regimen alone as a first-line treatment for advanced NSCLC.
Keywords: camrelizumab; chemotherapy; cost-utility analysis; first-line treatment; nonsquamous NSCLC.
Copyright © 2022 Chen, Xie, Zhao, Cai and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cost-effectiveness of camrelizumab plus chemotherapy vs. chemotherapy in the first-line treatment of non-squamous NSCLC: Evidence from China.Front Med (Lausanne). 2023 Feb 14;10:1122731. doi: 10.3389/fmed.2023.1122731. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36865055 Free PMC article.
-
Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China.Front Public Health. 2022 Aug 15;10:912921. doi: 10.3389/fpubh.2022.912921. eCollection 2022. Front Public Health. 2022. PMID: 36045725 Free PMC article.
-
Cost-Effectiveness Analysis of Camrelizumab Plus Chemotherapy vs. Chemotherapy Alone as the First-Line Treatment in Patients With IIIB-IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without EGFR and ALK Alteration from a Perspective of Health - Care System in China.Front Pharmacol. 2021 Dec 24;12:735536. doi: 10.3389/fphar.2021.735536. eCollection 2021. Front Pharmacol. 2021. PMID: 35002693 Free PMC article.
-
Economic Evaluation of First-Line Camrelizumab for Advanced Non-small-cell Lung Cancer in China.Front Public Health. 2021 Dec 10;9:743558. doi: 10.3389/fpubh.2021.743558. eCollection 2021. Front Public Health. 2021. PMID: 34957008 Free PMC article.
-
Cost-Effectiveness of Domestic PD-1 Inhibitor Camrelizumab Combined With Chemotherapy in the First-Line Treatment of Advanced Nonsquamous Non-Small-Cell Lung Cancer in China.Front Pharmacol. 2021 Nov 2;12:728440. doi: 10.3389/fphar.2021.728440. eCollection 2021. Front Pharmacol. 2021. PMID: 34795580 Free PMC article.
Cited by
-
Economic evaluation of five first-line PD-(L)1 inhibitors for treating non-squamous non-small cell lung cancer in China: A cost-effectiveness analysis based on network meta-analysis.Front Pharmacol. 2023 Mar 20;14:1119906. doi: 10.3389/fphar.2023.1119906. eCollection 2023. Front Pharmacol. 2023. PMID: 37021058 Free PMC article.
-
Clinical efficacy of Camrelizumab combined with first-line chemotherapy in extensive-stage small-cell lung cancer.Heliyon. 2023 Nov 28;10(1):e22913. doi: 10.1016/j.heliyon.2023.e22913. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38148793 Free PMC article.
-
Toripalimab plus chemotherapy vs. chemotherapy in patients with advanced non-small-cell lung cancer: A cost-effectiveness analysis.Front Pharmacol. 2023 Feb 14;14:1131219. doi: 10.3389/fphar.2023.1131219. eCollection 2023. Front Pharmacol. 2023. PMID: 36865925 Free PMC article.
-
Cost-effectiveness of camrelizumab plus chemotherapy vs. chemotherapy in the first-line treatment of non-squamous NSCLC: Evidence from China.Front Med (Lausanne). 2023 Feb 14;10:1122731. doi: 10.3389/fmed.2023.1122731. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36865055 Free PMC article.
-
Overexpression of TWF1 promotes lung adenocarcinoma progression and is associated with poor prognosis in cancer patients through the MMP1 signaling pathway.J Thorac Dis. 2023 May 30;15(5):2644-2658. doi: 10.21037/jtd-23-395. Epub 2023 May 22. J Thorac Dis. 2023. PMID: 37324107 Free PMC article.
References
-
- Shi J, Wang L, Wu N, Li J, Hui Z, Liu S, et al. . Clinical Characteristics and Medical Service Utilization of Lung Cancer in China, 2005-2014: Overall Design and Results From a Multicenter Retrospective Epidemiologic Survey. Lung Cancer (2019) 128:91–100. doi: 10.1016/j.lungcan.2018.11.031 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

