Alpha2beta1 Integrin Polymorphism in Diffuse Astrocytoma Patients
- PMID: 35936750
- PMCID: PMC9353741
- DOI: 10.3389/fonc.2022.914156
Alpha2beta1 Integrin Polymorphism in Diffuse Astrocytoma Patients
Abstract
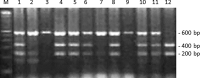
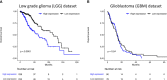
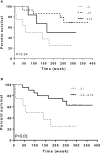
Integrins are heterodimeric transmembrane glycoproteins resulting from the non-covalent association of an α and β chain. The major integrin receptor for collagen/laminin, α2β1 is expressed on a wide variety of cell types and plays an essential role in the adhesion of normal and tumor cells to the extracellular matrix. Integrin-triggered signaling pathways promote the invasion and survival of glioma cells by modifying the brain microenvironment. In this study, we investigated the association of a specific genetic polymorphism of integrin α2β1 with the incidence of diffusely infiltrating astrocytoma and the progression of these tumors. Single-nucleotide polymorphism in intron 7 of the integrin ITGA2 gene was examined in 158 patients and 162 controls using polymerase chain reaction and restriction enzyme analysis. The ITGA2 genotype +/+ (with a BglII restriction site in both alleles) exhibited higher frequency in grade II astrocytoma compared to control (P = 0.02) whereas the genotype -/- (lacking the BglII site) correlated with the poorest survival rate (P = 0.04). In addition, in silico analyses of ITGA2 expression from low-grade gliomas (LGG, n = 515) and glioblastomas (GBM, n = 159) indicated that the higher expression of ITGA2 in LGG was associated with poor overall survival (P < 0.0001). However, the distribution of integrin ITGA2 BglII genotypes (+/+, +/-, -/-) was not significantly different between astrocytoma subgroups III and IV (P = 0.65, 0.24 and 0.33; 0.29, 0.48, 0.25, respectively) compared to control. These results suggest a narrow association between the presence of this SNP and indicate that further studies with larger samples are warranted to analyze the relation between tumor grade and overall survival, highlighting the importance of determining these polymorphisms for prognosis of astrocytomas.
Keywords: ITGA2; brain microenvironment; extracellular matrix; invasion; low grade glioma; single nucleotide polymorphism; tumor progression.
Copyright © 2022 Teixeira, Burim, Viapiano, Bidinotto, Nagashi Marie, Fleury Malheiros, Oba-Shinjo, Andrade and Carlotti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Association of BglII Polymorphism in ITGA2 and (894G/T and -786T/C) Polymorphisms in eNOS Gene With Stroke Susceptibility in Tunisian Patients α2 Gene Polymorphism in α2β1 Integrin and eNOS Gene Variants and Stroke.Biol Res Nurs. 2021 Jul;23(3):408-417. doi: 10.1177/1099800420977685. Epub 2020 Dec 10. Biol Res Nurs. 2021. PMID: 33297767
-
Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice.Matrix Biol. 2014 Feb;34:13-21. doi: 10.1016/j.matbio.2014.01.006. Epub 2014 Jan 27. Matrix Biol. 2014. PMID: 24480069
-
Integrin alpha-2 and beta-3 gene polymorphisms and breast cancer risk.Breast Cancer Res Treat. 2006 May;97(1):67-72. doi: 10.1007/s10549-005-9089-4. Breast Cancer Res Treat. 2006. PMID: 16317580
-
Association of platelet glycoprotein receptor alpha2beta1 integrin and glycoprotein IIIa gene polymorphisms with diabetic retinopathy: evidence from 3007 subjects.Curr Eye Res. 2015 May;40(5):476-83. doi: 10.3109/02713683.2014.932386. Epub 2014 Jun 30. Curr Eye Res. 2015. PMID: 24979111 Review.
-
Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas.J Neuropathol Exp Neurol. 2005 Jun;64(6):479-89. doi: 10.1093/jnen/64.6.479. J Neuropathol Exp Neurol. 2005. PMID: 15977639 Review.
References
-
- Ruiz–Ontanãon P, Orgaz JL, Aldaz B, Elosegui–Artola A, Martino J, Berciano MT, Montero JA, et al. . Cellular Plasticity Confers Migratory and Invasive Advantages to a Population of Glioblastoma–Initiating Cells That Infiltrate Peritumoral Tissue. Stem Cells (2013) 31:1075–85. doi: 10.1002/stem.1349 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

