Current Perspectives on Nucleos(t)ide Analogue Therapy for the Long-Term Treatment of Hepatitis B Virus
- PMID: 35936810
- PMCID: PMC9346298
- DOI: 10.2147/HMER.S291976
Current Perspectives on Nucleos(t)ide Analogue Therapy for the Long-Term Treatment of Hepatitis B Virus
Abstract
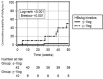
The hepatitis B virus (HBV) infection remains a global public health problem. This review presents updated recommendations for the optimal current treatment of choice with nucleos(t)ide analogues (NA). Current clinical practice guidelines on the management of chronic hepatitis B (CHB) by the Asian Pacific Association for the Study of the Liver, the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases have been considered. Patients with chronic HBV infection are at increased risk of liver disease progression to cirrhosis and hepatocellular carcinoma (HCC) development. The main goal of therapy is to improve survival preventing disease progression and HCC. The induction of long-term suppression of HBV replication represents the main endpoint of current treatment strategies, while hepatitis B surface antigen (HBsAg) loss is the optimal endpoint. The typical indication for treatment requires elevated HBV desoxyribonucleic acid (DNA), elevated alanine aminotransferase and/or at least moderate histological lesions, while all cirrhotic patients with detectable HBV DNA should be treated. The long-term administration of a potent NA with high barrier to resistance, ie, entecavir, tenofovir disoproxil fumarate or tenofovir alafenamide, represents the treatment of choice. However, HBsAg seroclearance is anecdotal with NA. Treated patients should be monitored for therapy response, adherence, risk of disease progression, and risk of HCC development. This review aims to assess the evolving trends on the potent NA and the new perspectives on finite therapy.
Keywords: HBsAg loss; antiviral therapy; efficacy; kinetics; treatment cessation.
© 2022 Broquetas and Carrión.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection.J Hepatol. 2017 Aug;67(2):370-398. doi: 10.1016/j.jhep.2017.03.021. Epub 2017 Apr 18. J Hepatol. 2017. PMID: 28427875
-
HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues.J Hepatol. 2019 Mar;70(3):361-370. doi: 10.1016/j.jhep.2018.10.014. Epub 2018 Oct 25. J Hepatol. 2019. PMID: 30367899
-
Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B.Liver Int. 2020 Jul;40(7):1578-1589. doi: 10.1111/liv.14482. Epub 2020 Apr 30. Liver Int. 2020. PMID: 32304611
-
INASL Position Statements on Prevention, Diagnosis and Management of Hepatitis B Virus Infection in India: The Andaman Statements.J Clin Exp Hepatol. 2018 Mar;8(1):58-80. doi: 10.1016/j.jceh.2017.12.001. Epub 2017 Dec 16. J Clin Exp Hepatol. 2018. PMID: 29743798 Free PMC article. Review.
-
Treatment of chronic hepatitis B virus infection - Dutch national guidelines.Neth J Med. 2008 Jul-Aug;66(7):292-306. Neth J Med. 2008. PMID: 18663260 Review.
Cited by
-
Hepatitis B Therapeutic Vaccine: A Patent Review.Pharmaceuticals (Basel). 2022 Dec 12;15(12):1542. doi: 10.3390/ph15121542. Pharmaceuticals (Basel). 2022. PMID: 36558991 Free PMC article.
-
Real-World Setting of Efficacy and Safety of 3 Years of Rifaximin Administration in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study.J Clin Med. 2025 Feb 18;14(4):1358. doi: 10.3390/jcm14041358. J Clin Med. 2025. PMID: 40004887 Free PMC article.
-
Treatment Discontinuation and Adherence in Patients With Chronic Hepatitis B Infection Newly Initiating Nucleos(t)ide Analogues in Japan: A Retrospective Cohort Study.J Viral Hepat. 2025 Sep;32(9):e70062. doi: 10.1111/jvh.70062. J Viral Hepat. 2025. PMID: 40810215 Free PMC article.
-
Combination therapy of therapeutic antibody and vaccine or entecavir in HBV carrier mice.Front Microbiol. 2023 May 5;14:1173061. doi: 10.3389/fmicb.2023.1173061. eCollection 2023. Front Microbiol. 2023. PMID: 37213494 Free PMC article.
-
Long-term entecavir therapy of chronic hepatitis B in real-life setting-Importance of quantitative HBsAg level.Indian J Gastroenterol. 2024 Jun;43(3):652-659. doi: 10.1007/s12664-023-01480-3. Epub 2023 Dec 30. Indian J Gastroenterol. 2024. PMID: 38158544
References
-
- World Health Organization (WHO). Global hepatitis report, 2017; 2017: 83.
Publication types
LinkOut - more resources
Full Text Sources