Skeletal muscle and metabolic flexibility in response to changing energy demands in wild birds
- PMID: 35936893
- PMCID: PMC9353400
- DOI: 10.3389/fphys.2022.961392
Skeletal muscle and metabolic flexibility in response to changing energy demands in wild birds
Abstract
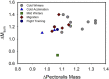
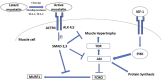
Phenotypically plastic responses of animals to adjust to environmental variation are pervasive. Reversible plasticity (i.e., phenotypic flexibility), where adult phenotypes can be reversibly altered according to prevailing environmental conditions, allow for better matching of phenotypes to the environment and can generate fitness benefits but may also be associated with costs that trade-off with capacity for flexibility. Here, we review the literature on avian metabolic and muscle plasticity in response to season, temperature, migration and experimental manipulation of flight costs, and employ an integrative approach to explore the phenotypic flexibility of metabolic rates and skeletal muscle in wild birds. Basal (minimum maintenance metabolic rate) and summit (maximum cold-induced metabolic rate) metabolic rates are flexible traits in birds, typically increasing with increasing energy demands. Because skeletal muscles are important for energy use at the organismal level, especially to maximum rates of energy use during exercise or shivering thermogenesis, we consider flexibility of skeletal muscle at the tissue and ultrastructural levels in response to variations in the thermal environment and in workloads due to flight exercise. We also examine two major muscle remodeling regulatory pathways: myostatin and insulin-like growth factor -1 (IGF-1). Changes in myostatin and IGF-1 pathways are sometimes, but not always, regulated in a manner consistent with metabolic rate and muscle mass flexibility in response to changing energy demands in wild birds, but few studies have examined such variation so additional study is needed to fully understand roles for these pathways in regulating metabolic flexibility in birds. Muscle ultrastrutural variation in terms of muscle fiber diameter and associated myonuclear domain (MND) in birds is plastic and highly responsive to thermal variation and increases in workload, however, only a few studies have examined ultrastructural flexibility in avian muscle. Additionally, the relationship between myostatin, IGF-1, and satellite cell (SC) proliferation as it relates to avian muscle flexibility has not been addressed in birds and represents a promising avenue for future study.
Keywords: IGF-1; hypertrophy; muscle; myonuclear domain; myostatin.
Copyright © 2022 Swanson, Zhang and Jimenez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

