Mu-opioid receptor-dependent transformation of respiratory motor pattern in neonates in vitro
- PMID: 35936900
- PMCID: PMC9353126
- DOI: 10.3389/fphys.2022.921466
Mu-opioid receptor-dependent transformation of respiratory motor pattern in neonates in vitro
Abstract
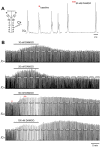
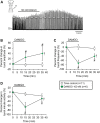
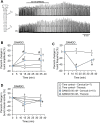
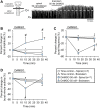
Endogenous opioid peptides activating mu-opioid receptors (MORs) are part of an intricate neuromodulatory system that coordinates and optimizes respiratory motor output to maintain blood-gas homeostasis. MOR activation is typically associated with respiratory depression but also has excitatory effects on breathing and respiratory neurons. We hypothesized that low level MOR activation induces excitatory effects on the respiratory motor pattern. Thus, low concentrations of an MOR agonist drug (DAMGO, 10-200 nM) were bath-applied to neonatal rat brainstem-spinal cord preparations while recording inspiratory-related motor output on cervical spinal roots (C4-C5). Bath-applied DAMGO (50-200 nM) increased inspiratory motor burst amplitude by 40-60% during (and shortly following) drug application with decreased burst frequency and minute activity. Reciprocal changes in inspiratory burst amplitude and frequency were balanced such that 20 min after DAMGO (50-200 nM) application, minute activity was unaltered compared to pre-DAMGO levels. The DAMGO-induced inspiratory burst amplitude increase did not require crossed cervical spinal pathways, was expressed on thoracic ventral spinal roots (T4-T8) and remained unaltered by riluzole pretreatment (blocks persistent sodium currents associated with gasping). Split-bath experiments showed that the inspiratory burst amplitude increase was induced only when DAMGO was bath-applied to the brainstem and not the spinal cord. Thus, MOR activation in neonates induces a respiratory burst amplitude increase via brainstem-specific mechanisms. The burst amplitude increase counteracts the expected MOR-dependent frequency depression and may represent a new mechanism by which MOR activation influences respiratory motor output.
Keywords: in vitro; mu-opiod receptor; neonatal rat; neuromodulation; respiratory motor control.
Copyright © 2022 Gumnit, Watters, Baker, Johnson and Johnson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Disinhibition does not play a role in endomorphin-2-induced changes in inspiratory motoneuron output produced by in vitro neonatal rat preparations.Respir Physiol Neurobiol. 2024 Feb;320:104186. doi: 10.1016/j.resp.2023.104186. Epub 2023 Nov 7. Respir Physiol Neurobiol. 2024. PMID: 37944625 Free PMC article.
-
Delta-opioid receptor activation prolongs respiratory motor output during oxygen-glucose deprivation in neonatal rat spinal cord in vitro.Neuroscience. 2011 Jul 28;187:70-83. doi: 10.1016/j.neuroscience.2011.04.059. Epub 2011 May 6. Neuroscience. 2011. PMID: 21571044 Free PMC article.
-
Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output.Neuroscience. 2006 Oct 27;142(3):885-92. doi: 10.1016/j.neuroscience.2006.06.036. Epub 2006 Aug 7. Neuroscience. 2006. PMID: 16893610
-
Biphasic effects of substance P on respiratory activity and respiration-related neurones in ventrolateral medulla in the neonatal rat brainstem in vitro.Acta Physiol Scand. 2002 Jan;174(1):67-84. doi: 10.1046/j.1365-201x.2002.00926.x. Acta Physiol Scand. 2002. PMID: 11851598
-
Current research in pathophysiology of opioid-induced respiratory depression, neonatal opioid withdrawal syndrome, and neonatal antidepressant exposure syndrome.Curr Res Toxicol. 2022 Jun 6;3:100078. doi: 10.1016/j.crtox.2022.100078. eCollection 2022. Curr Res Toxicol. 2022. PMID: 35734228 Free PMC article.
Cited by
-
Endomorphin-2 (Endo2) and substance P (SubP) co-application attenuates SubP-induced excitation and alters frequency plasticity in neonatal rat in vitro preparations.Respir Physiol Neurobiol. 2025 Jan;331:104351. doi: 10.1016/j.resp.2024.104351. Epub 2024 Sep 19. Respir Physiol Neurobiol. 2025. PMID: 39303801
-
Maternal opioids age-dependently impair neonatal respiratory control networks.Front Physiol. 2023 Mar 16;14:1109754. doi: 10.3389/fphys.2023.1109754. eCollection 2023. Front Physiol. 2023. PMID: 37008014 Free PMC article.
-
Disinhibition does not play a role in endomorphin-2-induced changes in inspiratory motoneuron output produced by in vitro neonatal rat preparations.Respir Physiol Neurobiol. 2024 Feb;320:104186. doi: 10.1016/j.resp.2023.104186. Epub 2023 Nov 7. Respir Physiol Neurobiol. 2024. PMID: 37944625 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

