Review of data processing of functional optical microscopy for neuroscience
- PMID: 35937186
- PMCID: PMC9351186
- DOI: 10.1117/1.NPh.9.4.041402
Review of data processing of functional optical microscopy for neuroscience
Abstract
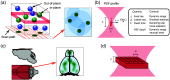
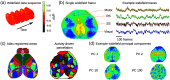
Functional optical imaging in neuroscience is rapidly growing with the development of optical systems and fluorescence indicators. To realize the potential of these massive spatiotemporal datasets for relating neuronal activity to behavior and stimuli and uncovering local circuits in the brain, accurate automated processing is increasingly essential. We cover recent computational developments in the full data processing pipeline of functional optical microscopy for neuroscience data and discuss ongoing and emerging challenges.
Keywords: calcium imaging; data analysis; fluorescence microscopy; functional imaging.
© 2022 The Authors.
Figures



Similar articles
-
An integrative approach for analyzing hundreds of neurons in task performing mice using wide-field calcium imaging.Sci Rep. 2016 Feb 8;6:20986. doi: 10.1038/srep20986. Sci Rep. 2016. PMID: 26854041 Free PMC article.
-
Advances in two photon scanning and scanless microscopy technologies for functional neural circuit imaging.Proc IEEE Inst Electr Electron Eng. 2017 Jan;105(1):139-157. doi: 10.1109/JPROC.2016.2577380. Epub 2016 Sep 28. Proc IEEE Inst Electr Electron Eng. 2017. PMID: 28757657 Free PMC article.
-
Monitoring activity in neural circuits with genetically encoded indicators.Front Mol Neurosci. 2014 Dec 5;7:97. doi: 10.3389/fnmol.2014.00097. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25538558 Free PMC article. Review.
-
Light sheet fluorescence microscopy for neuroscience.J Neurosci Methods. 2019 May 1;319:16-27. doi: 10.1016/j.jneumeth.2018.07.011. Epub 2018 Jul 23. J Neurosci Methods. 2019. PMID: 30048674 Review.
-
Advancing multiscale structural mapping of the brain through fluorescence imaging and analysis across length scales.Interface Focus. 2016 Feb 6;6(1):20150081. doi: 10.1098/rsfs.2015.0081. Interface Focus. 2016. PMID: 26855758 Free PMC article.
Cited by
-
Special Section Guest Editorial: Computational Approaches for Neuroimaging.Neurophotonics. 2022 Oct;9(4):041401. doi: 10.1117/1.NPh.9.4.041401. Epub 2022 Sep 2. Neurophotonics. 2022. PMID: 36062025 Free PMC article.
-
Fast Two-photon Microscopy by Neuroimaging with Oblong Random Acquisition (NORA).ArXiv [Preprint]. 2025 Jun 9:arXiv:2503.15487v2. ArXiv. 2025. PMID: 40166740 Free PMC article. Preprint.
-
Two-photon calcium imaging of neuronal activity.Nat Rev Methods Primers. 2022;2(1):67. doi: 10.1038/s43586-022-00147-1. Epub 2022 Sep 1. Nat Rev Methods Primers. 2022. PMID: 38124998 Free PMC article.
-
GraFT: Graph Filtered Temporal Dictionary Learning for Functional Neural Imaging.IEEE Trans Image Process. 2022;31:3509-3524. doi: 10.1109/TIP.2022.3171414. Epub 2022 May 18. IEEE Trans Image Process. 2022. PMID: 35533160 Free PMC article.
-
Modeling the diverse effects of divisive normalization on noise correlations.PLoS Comput Biol. 2023 Nov 30;19(11):e1011667. doi: 10.1371/journal.pcbi.1011667. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 38033166 Free PMC article.
References
-
- Takahashi D. Y., et al. , “Social-vocal brain networks in a non-human primate,” bioRxiv (2021).

