Serum Cannabinoid 24 h and 1 Week Steady State Pharmacokinetic Assessment in Cats Using a CBD/CBDA Rich Hemp Paste
- PMID: 35937287
- PMCID: PMC9355628
- DOI: 10.3389/fvets.2022.895368
Serum Cannabinoid 24 h and 1 Week Steady State Pharmacokinetic Assessment in Cats Using a CBD/CBDA Rich Hemp Paste
Abstract
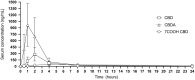
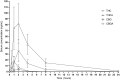
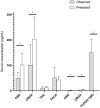
Hemp based cannabinoids have gained popularity in veterinary medicine due to the potential to treat pain, seizure disorders and dermatological maladies in dogs. Cat owners are also using hemp-based products for arthritis, anxiety and neoplastic disorders with no studies assessing hemp cannabinoids, namely cannabidiol efficacy, for such disorders. Initial twenty-four pharmacokinetic and chronic dosing serum concentration in cats are sparse. The aim of our study was to assess 8 cats physiological and 24 h and 1-week steady state pharmacokinetic response to a cannabidiol (CBD) and cannabidiolic acid (CBDA) rich hemp in a palatable oral paste. Using a standard dose of paste (6.4 mg/CBD + CBDA 5.3 mg/gram) across 8 cats weighing between 4.2 and 5.4 kg showed an average maximal concentration of CBD at 282.0 ± 149.4 ng/mL with a half-life of ~2.1 ± 1.1 h, and CBDA concentrations of 1,011.3 ± 495.4 ng/mL with a half-life of ~2.7 ± 1.4 h, showing superior absorption of CBDA. After twice daily dosing for 1 week the serum concentrations 6 h after a morning dosing showed that the acidic forms of the cannabinoids were approximately double the concentration of the non-acidic forms like CBD and Δ9- tetrahydrocannabinol (THC). The results of this study compared to two other recent studies suggest that the absorption in this specific paste product may be superior to oil bases used previously, and show that the acidic forms of cannabinoids appear to be absorbed better than the non-acidic forms. More importantly, physical and behavioral examinations every morning after dosing showed no adverse events related to neurological function or behavioral alterations. In addition, bloodwork after 1 week of treatment showed no clinically significant serum biochemical alterations as a reflection of hepatic and renal function all remaining within the reference ranges set by the diagnostic laboratory suggesting that short-term treatment was safe.
Keywords: cannabidiol; cannabidiolic acid; cannabinoid; feline; hemp (Cannabis sativa L.).
Copyright © 2022 Wang, Zakharov, Gomez, Lyubimov, Trottier, Schwark and Wakshlag.
Conflict of interest statement
JW and WS are paid consultants for Ellevet Sciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract.Front Vet Sci. 2020 Sep 4;7:505. doi: 10.3389/fvets.2020.00505. eCollection 2020. Front Vet Sci. 2020. PMID: 33102539 Free PMC article.
-
Chronic oral dosing of cannabidiol and cannabidiolic acid full-spectrum hemp oil extracts has no adverse effects in horses: a pharmacokinetic and safety study.Am J Vet Res. 2025 Jan 9;86(3):ajvr.24.08.0235. doi: 10.2460/ajvr.24.08.0235. Print 2025 Mar 1. Am J Vet Res. 2025. PMID: 39787699
-
Safety and efficacy of cannabidiol-cannabidiolic acid rich hemp extract in the treatment of refractory epileptic seizures in dogs.Front Vet Sci. 2022 Jul 29;9:939966. doi: 10.3389/fvets.2022.939966. eCollection 2022. Front Vet Sci. 2022. PMID: 35967998 Free PMC article.
-
Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer.Biomed Res Int. 2018 Dec 4;2018:1691428. doi: 10.1155/2018/1691428. eCollection 2018. Biomed Res Int. 2018. PMID: 30627539 Free PMC article. Review.
-
A cannabinoid Hairy-Tale: Hair loss or hair gain?J Cosmet Dermatol. 2022 Dec;21(12):6653-6660. doi: 10.1111/jocd.15427. Epub 2022 Oct 14. J Cosmet Dermatol. 2022. PMID: 36181341 Review.
Cited by
-
Tolerability of 2 and 4 mg/kg Dosing Every 12 Hour of a Cannabidiol- and Cannabidiolic Acid-Rich Hemp Extract on Mixed-Breed Dogs Utilized for Teaching in a Closed Colony.Animals (Basel). 2024 Jun 24;14(13):1863. doi: 10.3390/ani14131863. Animals (Basel). 2024. PMID: 38997975 Free PMC article.
-
Neuropathic pain in cats: Mechanisms and multimodal management.J Feline Med Surg. 2024 May;26(5):1098612X241246518. doi: 10.1177/1098612X241246518. J Feline Med Surg. 2024. PMID: 38710218 Free PMC article. Review.
-
Disposition of a single oral dose of a cannabidiol medication in healthy cats.Front Vet Sci. 2023 May 26;10:1181517. doi: 10.3389/fvets.2023.1181517. eCollection 2023. Front Vet Sci. 2023. PMID: 37303724 Free PMC article.
-
Cannabidiol as an immune modulator: A comprehensive review.Saudi Pharm J. 2025 May 23;33(3):11. doi: 10.1007/s44446-025-00005-7. Saudi Pharm J. 2025. PMID: 40407987 Free PMC article. Review.
-
Endocannabinoid system and phytocannabinoids in the main species of veterinary interest: a comparative review.Vet Res Commun. 2024 Oct;48(5):2915-2941. doi: 10.1007/s11259-024-10509-7. Epub 2024 Aug 20. Vet Res Commun. 2024. PMID: 39162768 Free PMC article. Review.
References
-
- Jin J-J, Yang M-Q, Fritsch PW, van Velzen R, Li D-Z, Yi T-S. Born migrators: historical biogeography of the cosmopolitan family Cannabaceae. J System Evol. (2020) 58:461–73. 10.1111/jse.12552 - DOI
-
- National National Academies of Sciences E Division HM Practice Practice B on PH PH Agenda Agenda C on the HE of MAER R. Therapeutic Effects of Cannabis and Cannabinoids. National Academies Press. (2017). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK425767/ (accessed October 5, 2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

