The Combination of Radiotherapy and Complement C3a Inhibition Potentiates Natural Killer cell Functions Against Pancreatic Cancer
- PMID: 35937458
- PMCID: PMC9354534
- DOI: 10.1158/2767-9764.crc-22-0069
The Combination of Radiotherapy and Complement C3a Inhibition Potentiates Natural Killer cell Functions Against Pancreatic Cancer
Abstract
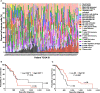
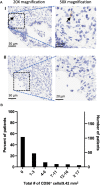
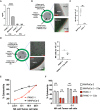
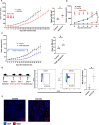
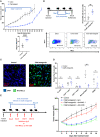
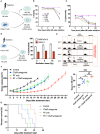
Pancreatic cancer is one of the deadliest cancers, against which current immunotherapy strategies are not effective. Herein, we analyzed the immune cell composition of the tumor microenvironment of pancreatic cancer samples in The Cancer Genome Atlas and found that the presence of intratumoral NK cells correlates with survival. Subsequent analysis also indicated that NK cell exclusion from the microenvironment is found in a high percentage of clinical pancreatic cancers and in preclinical models of pancreatic cancer. Mechanistically, NK cell exclusion is regulated in part by complement C3a and its receptor signaling. Inhibition of the C3a receptor enhances NK cell infiltration in syngeneic mouse models of pancreatic cancer resulting in tumor growth delay. However, tumor growth inhibition mediated by NK cells is not sufficient alone for complete tumor regression, but is potentiated when combined with radiation therapy. Our findings indicate that although C3a inhibition is a promising approach to enhance NK cell-based immunotherapy against pancreatic cancer, its combination with radiation therapy hold greater therapeutic benefit.
Conflict of interest statement
M.M. Olcina reports grants from Medical Research Council Unit Grant and Cancer Research UK Oxford Centre during the conduct of the study. Q.-T. Le reports non-financial support from Merck; personal fees from Nanobiotix, Roche, and Coherus outside the submitted work. A.J. Giaccia reports grants from NCI and ASCO during the conduct of the study. No disclosures were reported by the other authors.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Pourshams A, Sepanlou SG, Ikuta KS, Bisignano C, Safiri S, Roshandel G, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:934–47. - PMC - PubMed
-
- Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology 2021;160:744–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

