Vaginal microecological characteristics of women in different physiological and pathological period
- PMID: 35937699
- PMCID: PMC9354832
- DOI: 10.3389/fcimb.2022.959793
Vaginal microecological characteristics of women in different physiological and pathological period
Abstract
The vaginal microbiota, the host endocrine system, the vaginal anatomy, and the local mucosal immunity comprise the vaginal microbiota, which interacts with each other to maintain the balance of the vaginal microbiota, which maintains female reproductive health. Puberty, menstruation, pregnancy, and menopause are four phases women go through during their reproductive and post-reproductive years. Vaginal microbiota composition and abundance are heavily influenced by estrogen and progesterone, which start at puberty and continue during the reproductive years in a dynamic balance with some fluctuations. Estrogen promotes proliferation of vaginal epithelial cells and increases glycogen storage, while progesterone lyses vaginal epithelial cells, facilitating the release of glycogen to maintain normal pH. This review summarizes the latest national and international evidence on the composition and distribution of vaginal microecology in women during different physiological and pathological periods and proposes a hormone-driven microbial diversity hypothesis to explain the temporal patterns of vaginal microbial diversity during the female reproductive cycle and menopause. A relatively balanced vaginal microecological system has a positive effect on the maintenance of female health. An imbalance in the ratio of flora can lead to susceptibility to infections or reproductive complications. The study of human microecology and its role in the development and progression of human disease is essential for the prevention, diagnosis, and treatment of related obstetric and gynecologic conditions.
Keywords: cancer; inflammation; reproductive health; vaginal microbiota; vaginal microecological.
Copyright © 2022 Shen, Zhang, Yuan, Zhu and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
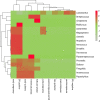
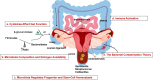
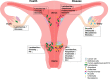
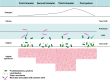
References
-
- Allen N. E., Key T. J., Dossus L., Rinaldi S., Cust A., Lukanova A., et al. . (2008). Endogenous sex hormones and endometrial cancer risk in women in the European prospective investigation into cancer and nutrition (EPIC). Endocr. Relat. Cancer 15 (2), 485–497. doi: 10.1677/ERC-07-0064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

