Development and therapeutic manipulation of the head and neck cancer tumor environment to improve clinical outcomes
- PMID: 35937775
- PMCID: PMC9354490
- DOI: 10.3389/froh.2022.902160
Development and therapeutic manipulation of the head and neck cancer tumor environment to improve clinical outcomes
Abstract
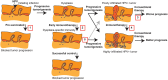
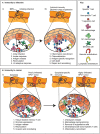
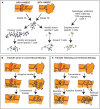
The clinical response to cancer therapies involves the complex interplay between the systemic, tumoral, and stromal immune response as well as the direct impact of treatments on cancer cells. Each individual's immunological and cancer histories are different, and their carcinogen exposures may differ. This means that even though two patients with oral tumors may carry an identical mutation in TP53, they are likely to have different pre-existing immune responses to their tumors. These differences may arise due to their distinct accessory mutations, genetic backgrounds, and may relate to clinical factors including previous chemotherapy exposure and concurrent medical comorbidities. In isolation, their cancer cells may respond similarly to cancer therapy, but due to their baseline variability in pre-existing immune responses, patients can have different responses to identical therapies. In this review we discuss how the immune environment of tumors develops, the critical immune cell populations in advanced cancers, and how immune interventions can manipulate the immune environment of patients with pre-malignancies or advanced cancers to improve therapeutic outcomes.
Keywords: CD4; CD8; HNSCC (head and neck squamous cell carcinoma); OSCC (oral squamous cell carcinoma); TIL (tumor infiltrating lymphocytes); immunotherapy; pre-malignancies.
Copyright © 2022 Duhen, Gough, Leidner and Stanton.
Figures

Similar articles
-
The differences of immunologic and TP53 mutant phenotypes between synchronous and metachronous head and neck cancer and esophageal cancer.Oral Oncol. 2020 Dec;111:104945. doi: 10.1016/j.oraloncology.2020.104945. Epub 2020 Aug 5. Oral Oncol. 2020. PMID: 32769036
-
Impact of HPV status on immune responses in head and neck squamous cell carcinoma.Oral Oncol. 2022 Apr;127:105774. doi: 10.1016/j.oraloncology.2022.105774. Epub 2022 Feb 24. Oral Oncol. 2022. PMID: 35219073
-
Expression of immune response biomarkers (PD‑L1, p16, CD3+ and CD8+ TILs) in recurrent head and neck squamous cell carcinoma within previously irradiated areas.Oncol Rep. 2021 Mar;45(3):1273-1283. doi: 10.3892/or.2021.7928. Epub 2021 Jan 7. Oncol Rep. 2021. PMID: 33432367
-
Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer.Oral Oncol. 2016 Oct;61:159-65. doi: 10.1016/j.oraloncology.2016.08.003. Epub 2016 Aug 21. Oral Oncol. 2016. PMID: 27553942 Free PMC article. Review.
-
Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments.Semin Cancer Biol. 2018 Oct;52(Pt 2):228-240. doi: 10.1016/j.semcancer.2018.01.008. Epub 2018 Jan 31. Semin Cancer Biol. 2018. PMID: 29355614 Review.
Cited by
-
Deciphering the role of HPV-mediated metabolic regulation in shaping the tumor microenvironment and its implications for immunotherapy in HNSCC.Front Immunol. 2023 Oct 9;14:1275270. doi: 10.3389/fimmu.2023.1275270. eCollection 2023. Front Immunol. 2023. PMID: 37876923 Free PMC article. Review.
-
Tumor resident memory CD8 T cells and concomitant tumor immunity develop independently of CD4 help.Sci Rep. 2023 Apr 18;13(1):6277. doi: 10.1038/s41598-023-33508-1. Sci Rep. 2023. PMID: 37072485 Free PMC article.
References
-
- Danilova L, Anagnostou V, Caushi JX, Sidhom JW, Guo H, Chan HY, et al. . The mutation-associated neoantigen functional expansion of specific T cells (MANAFEST) assay: a sensitive platform for monitoring antitumor immunity. Cancer Immunol Res. (2018) 6:888–99. 10.1158/2326-6066.CIR-18-0129 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

