Alterations in Spontaneous Neuronal Activity and Microvascular Density of the Optic Nerve Head in Active Thyroid-Associated Ophthalmopathy
- PMID: 35937801
- PMCID: PMC9354054
- DOI: 10.3389/fendo.2022.895186
Alterations in Spontaneous Neuronal Activity and Microvascular Density of the Optic Nerve Head in Active Thyroid-Associated Ophthalmopathy
Abstract
Purpose: To investigate changes in local spontaneous brain activity in patients with active thyroid-associated ophthalmopathy (TAO) and explore the relationship between such alterations and microvascular indices.
Methods: Thirty-six active TAO patients with active phase and 39 healthy controls (HCs) were enrolled in this study. All participants underwent resting-state functional magnetic resonance imaging (rs-fMRI), neuropsychological tests, and ophthalmological examinations. The rs-fMRI-based fractional low-frequency fluctuation amplitude (fALFF) analysis methods were used to assess spontaneous brain activity in both groups. The structure (peripapillary retinal nerve fiber layer, pRNFL) and microvascular indices (the optic nerve head (ONH) whole image vessel density, ONH-wiVD, and peripapillary vessel density) were analyzed through optical coherence tomographic angiography imaging. The relationship between abnormal spontaneous brain activity and ophthalmological indices was analyzed using the Spearman's rank correlation analysis.
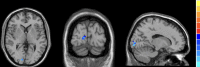
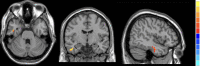
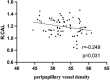
Results: Compared with HCs, active TAO patients had increased fALFF in the right inferior temporal gyrus (R.ITG) and left posterior cingulate gyrus (L.PCC), but decreased fALFF in the right calcarine (R.CAL). The fALFF values in L.PCC were positively correlated with peripapillary vessel density, whereas fALFF values in R.CAL were negatively related to peripapillary vessel density.
Conclusions: This study demonstrates that changes in spontaneous brain activity of active TAO are accompanied by peripapillary microvascular variations. These results provide insights into the pathophysiological mechanisms of active TAO. In addition, the combination of fALFF values and peripapillary vessel density may be served as important references for better clinical decision making.
Keywords: active thyroid-associated ophthalmopathy; fMRI; fractional low-frequency fluctuation amplitude; optical coherence tomography angiography; resting state.
Copyright © 2022 Zhu, Liu, Lu, Wang, Zhang, Zhao, Lin, Hussein, Liu, Yan, Bai and Tu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Blum Meirovitch S, Leibovitch I, Kesler A, Varssano D, Rosenblatt A, Neudorfer M. Retina and Nerve Fiber Layer Thickness in Eyes With Thyroid-Associated Ophthalmopathy. Isr Med Assoc J (2017) 19:277–81. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

