Reduced Ca2+ transient amplitudes may signify increased or decreased depolarization depending on the neuromodulatory signaling pathway
- PMID: 35937887
- PMCID: PMC9354622
- DOI: 10.3389/fnins.2022.931328
Reduced Ca2+ transient amplitudes may signify increased or decreased depolarization depending on the neuromodulatory signaling pathway
Abstract
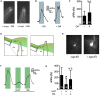
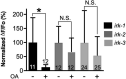
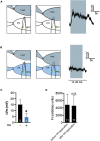
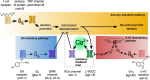
Neuromodulators regulate neuronal excitability and bias neural circuit outputs. Optical recording of neuronal Ca2+ transients is a powerful approach to study the impact of neuromodulators on neural circuit dynamics. We are investigating the polymodal nociceptor ASH in Caenorhabditis elegans to better understand the relationship between neuronal excitability and optically recorded Ca2+ transients. ASHs depolarize in response to the aversive olfactory stimulus 1-octanol (1-oct) with a concomitant rise in somal Ca2+, stimulating an aversive locomotory response. Serotonin (5-HT) potentiates 1-oct avoidance through Gαq signaling, which inhibits L-type voltage-gated Ca2+ channels in ASH. Although Ca2+ signals in the ASH soma decrease, depolarization amplitudes increase because Ca2+ mediates inhibitory feedback control of membrane potential in this context. Here, we investigate octopamine (OA) signaling in ASH to assess whether this negative correlation between somal Ca2+ and depolarization amplitudes is a general phenomenon, or characteristic of certain neuromodulatory pathways. Like 5-HT, OA reduces somal Ca2+ transient amplitudes in ASH neurons. However, OA antagonizes 5-HT modulation of 1-oct avoidance behavior, suggesting that OA may signal through a different pathway. We further show that the pathway for OA diminution of ASH somal Ca2+ consists of the OCTR-1 receptor, the Go heterotrimeric G-protein, and the G-protein activated inwardly rectifying channels IRK-2 and IRK-3, and this pathway reduces depolarization amplitudes in parallel with somal Ca2+ transient amplitudes. Therefore, even within a single neuron, somal Ca2+ signal reduction may indicate either increased or decreased depolarization amplitude, depending on which neuromodulatory signaling pathways are activated, underscoring the need for careful interpretation of Ca2+ imaging data in neuromodulatory studies.
Keywords: Ca2+ dynamics; depolarization; graded potentials; neuromodulation; signal integration.
Copyright © 2022 Debnath, Williams and Bamber.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
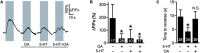
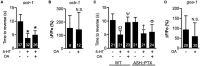

Similar articles
-
Serotonin Disinhibits a Caenorhabditis elegans Sensory Neuron by Suppressing Ca2+-Dependent Negative Feedback.J Neurosci. 2018 Feb 21;38(8):2069-2080. doi: 10.1523/JNEUROSCI.1908-17.2018. Epub 2018 Jan 22. J Neurosci. 2018. PMID: 29358363 Free PMC article.
-
Serotonin differentially modulates Ca2+ transients and depolarization in a C. elegans nociceptor.J Neurophysiol. 2015 Feb 15;113(4):1041-50. doi: 10.1152/jn.00665.2014. Epub 2014 Nov 19. J Neurophysiol. 2015. PMID: 25411461 Free PMC article.
-
The G protein regulators EGL-10 and EAT-16, the Giα GOA-1 and the G(q)α EGL-30 modulate the response of the C. elegans ASH polymodal nociceptive sensory neurons to repellents.BMC Biol. 2010 Nov 11;8:138. doi: 10.1186/1741-7007-8-138. BMC Biol. 2010. PMID: 21070627 Free PMC article.
-
Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain.Croat Med J. 2007 Feb;48(1):9-21. Croat Med J. 2007. PMID: 17309135 Free PMC article. Review.
-
Regulation of synaptic signalling by postsynaptic, non-glutamate receptor ion channels.J Physiol. 2008 Mar 15;586(6):1475-80. doi: 10.1113/jphysiol.2007.148353. Epub 2007 Dec 20. J Physiol. 2008. PMID: 18096597 Free PMC article. Review.
Cited by
-
Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model.Cells. 2023 Apr 13;12(8):1148. doi: 10.3390/cells12081148. Cells. 2023. PMID: 37190057 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

