The impact of maternal high-fat diet on offspring neurodevelopment
- PMID: 35937892
- PMCID: PMC9354026
- DOI: 10.3389/fnins.2022.909762
The impact of maternal high-fat diet on offspring neurodevelopment
Abstract
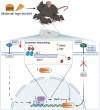
A maternal high-fat diet affects offspring neurodevelopment with long-term consequences on their brain health and behavior. During the past three decades, obesity has rapidly increased in the whole human population worldwide, including women of reproductive age. It is known that maternal obesity caused by a high-fat diet may lead to neurodevelopmental disorders in their offspring, such as autism spectrum disorder, attention deficit hyperactivity disorder, anxiety, depression, and schizophrenia. A maternal high-fat diet can affect offspring neurodevelopment due to inflammatory activation of the maternal gut, adipose tissue, and placenta, mirrored by increased levels of pro-inflammatory cytokines in both maternal and fetal circulation. Furthermore, a maternal high fat diet causes gut microbial dysbiosis further contributing to increased inflammatory milieu during pregnancy and lactation, thus disturbing both prenatal and postnatal neurodevelopment of the offspring. In addition, global molecular and cellular changes in the offspring's brain may occur due to epigenetic modifications including the downregulation of brain-derived neurotrophic factor (BDNF) expression and the activation of the endocannabinoid system. These neurodevelopmental aberrations are reflected in behavioral deficits observed in animals, corresponding to behavioral phenotypes of certain neurodevelopmental disorders in humans. Here we reviewed recent findings from rodent models and from human studies to reveal potential mechanisms by which a maternal high-fat diet interferes with the neurodevelopment of the offspring.
Keywords: behavioral deficits; epigenetic regulation; gut microbiota; inflammation; maternal high-fat diet (mHFD); neurodevelopmental disorders.
Copyright © 2022 Urbonaite, Knyzeliene, Bunn, Smalskys and Neniskyte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Almeida M. M., Dias-Rocha C. P., Reis-Gomes C. F., Wang H., Atella G. C., Cordeiro A., et al. (2019). Maternal high-fat diet impairs leptin signaling and up-regulates type-1 cannabinoid receptor with sex-specific epigenetic changes in the hypothalamus of newborn rats. Psychoneuroendocrinology 103 306–315. 10.1016/j.psyneuen.2019.02.004 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

