WDR36 Safeguards Self-Renewal and Pluripotency of Human Extended Pluripotent Stem Cells
- PMID: 35937980
- PMCID: PMC9353684
- DOI: 10.3389/fgene.2022.905395
WDR36 Safeguards Self-Renewal and Pluripotency of Human Extended Pluripotent Stem Cells
Abstract
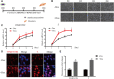
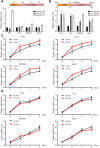
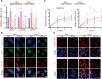
Extended pluripotent stem cells (EPS cells) have unlimited self-renewal ability and the potential to differentiate into mesodermal, ectodermal, and endodermal cells. Notably, in addition to developing the embryonic (Em) lineages, it can also make an effective contribution to extraembryonic (ExEm) lineages both in vitro and in vivo. However, multiple mysteries still remain about the underlying molecular mechanism of EPS cells' maintenance and developmental potential. WDR36 (WD Repeat Domain 36), a protein of 105 kDa with 14 WD40 repeats, which may fold into two β-propellers, participates in 18sRNA synthesis and P53 stress response. Though WDR36 safeguards mouse early embryonic development, that is, homozygous knockout of WDR36 can result in embryonic lethality, what role does WDR36 plays in self-renewal and differentiation developmental potential of human EPS cells is still a subject of concern. Here, our findings suggested that the expression of WDR36 was downregulated during human hEPS cells lost self-renewal. Through constructing inducible knockdown or overexpressing WDR36-human EPS cell lines, we found that WDR36 knockdown disrupted self-renewal but promoted the mesodermal differentiation of human EPS cells; however, overexpressing of WDR36 had little effect. Additionally, P53 inhibition could reverse the effects of WDR36 knockdown, on both self-renewal maintenance and differentiation potential of human EPS cells. These data implied that WDR36 safeguards self-renewal and pluripotency of human EPS cells, which would extend our understanding of the molecular mechanisms of human EPS cells' self-renewal and differentiation.
Keywords: WDR36; differentiation; hEPS cells; p53; self-renewal.
Copyright © 2022 An, Yao, Zhang, Sun, Yu, Jia and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
WDR36 Regulates Trophectoderm Differentiation During Human Preimplantation Embryonic Development Through Glycolytic Metabolism.Adv Sci (Weinh). 2025 Feb;12(5):e2412222. doi: 10.1002/advs.202412222. Epub 2024 Dec 10. Adv Sci (Weinh). 2025. PMID: 39656902 Free PMC article.
-
Lack of WDR36 leads to preimplantation embryonic lethality in mice and delays the formation of small subunit ribosomal RNA in human cells in vitro.Hum Mol Genet. 2011 Feb 1;20(3):422-35. doi: 10.1093/hmg/ddq478. Epub 2010 Nov 3. Hum Mol Genet. 2011. PMID: 21051332
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
-
Generation of pig induced pluripotent stem cells using an extended pluripotent stem cell culture system.Stem Cell Res Ther. 2019 Jun 27;10(1):193. doi: 10.1186/s13287-019-1303-0. Stem Cell Res Ther. 2019. PMID: 31248457 Free PMC article.
-
β-Catenin in pluripotency: adhering to self-renewal or Wnting to differentiate?Int Rev Cell Mol Biol. 2014;312:53-78. doi: 10.1016/B978-0-12-800178-3.00002-6. Int Rev Cell Mol Biol. 2014. PMID: 25262238 Review.
Cited by
-
WDR36 Regulates Trophectoderm Differentiation During Human Preimplantation Embryonic Development Through Glycolytic Metabolism.Adv Sci (Weinh). 2025 Feb;12(5):e2412222. doi: 10.1002/advs.202412222. Epub 2024 Dec 10. Adv Sci (Weinh). 2025. PMID: 39656902 Free PMC article.
References
-
- Blankenbach K. V., Bruno G., Wondra E., Spohner A. K., Aster N. J., Vienken H., et al. (2020). The WD40 Repeat Protein, WDR36, Orchestrates Sphingosine Kinase-1 Recruitment and Phospholipase C-β Activation by Gq-Coupled Receptors. Biochimica Biophysica Acta (BBA) - Mol. Cell. Biol. Lipids 1865, 158704. 10.1016/j.bbalip.2020.158704 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

