Visible-Light Photoinitiation of (Meth)acrylate Polymerization with Autonomous Post-conversion
- PMID: 35938043
- PMCID: PMC9351574
- DOI: 10.1021/acs.macromol.1c00761
Visible-Light Photoinitiation of (Meth)acrylate Polymerization with Autonomous Post-conversion
Abstract
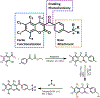
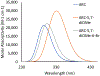
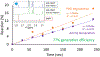
Conversion plateaus rapidly in radical photopolymerizations (RPPs) following discontinuation of irradiation due to rapid termination of reactive radicals, which restricts the wider use of RPPs in applications that involve nonuniform light access including those with attenuated light transmission or irregular surfaces. Based on our recent report of a radical dark-curing photoinitiator (DCPI) that continues polymerization beyond the cessation of irradiation by enabling latent redox initiation with photo-released amine in the presence of a suitable oxidant, we developed a new DCPI with an absorption spectrum that extends well into the visible range. Our design process involved a series of computational investigations of candidate molecules, including a systematic study of substituents and their position-dependent effects on absorption characteristics, electronic transitions, and the photochemical mechanism and its associated energetics. Our quantum chemical computations identified the target compound 5,7-dimethoxy-6-bromo-3-aroylcoumarin-DMPT/BPh4 and predicted that it would facilitate the dark-curing mechanism by concurrent photo-radical generation and photo-induced release of an efficient redox reductant under visible irradiation. This reductant-tethered chromophore was then synthesized and optically characterized with UV-vis spectroscopy that revealed its strong visible-light absorption with a molar absorptivity of 5710 M-1 cm-1 at 405 nm and 50 M-1 cm-1 at 455 nm. We then demonstrated extensive dark-curing of >35% additional conversion over 25 min following brief activation of the shelf-stable one-part system by irradiation with a 455 nm LED that was ceased at 20% conversion. In contrast, shuttering irradiation of the control formulation at that same point resulted in immediate cessation of conversion, which plateaued at 20%. We determined a remarkable initiator efficiency of 2.82 that results from the additional redox-generated radicals with a 77% photo-reductant generation quantum yield. The combination of superior photo- and dark-curing efficiencies of this new visible DCPI is expected to open new application opportunities in RPP, especially those involving resins that are highly light attenuating, surfaces that possess irregular features that produce uneven irradiance, and production lines where continued dark-curing downstream of the light activation step enhances line efficiencies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
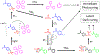
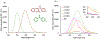
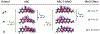



References
-
- Fouassier JP; Allonas X; Burget D Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coatings 2003, 47, 16–36.
-
- Marqual IT Solutions Pvt Ltd (KBV Research). Global UV Curable Resins Market Outlook to 2025. https://www.researchandmarkets.com/reports/4848974/global-uv-curable-res..., accessed July 21st, 2020
-
- Palin WM; Leprince JG; Hadis MA Shining a light on high volume photocurable materials. Dent. Mater 2018, 34, 695–710. - PubMed
-
- Garra P; Dietlin C; Morlet-Savary F; et al. Photopolymerization processes of thick films and in shadow areas: a review for the access to composites. Polym. Chem 2017, 8, 7088–7101.
-
- Kim K; Sinha J; Gao G; et al. High-Efficiency Radical Photopolymerization Enhanced by Autonomous Dark Cure. Macromolecules 2020, 53, 5034–5046.
Grants and funding
LinkOut - more resources
Full Text Sources
