The endoplasmic reticulum membrane complex promotes proteostasis of GABAA receptors
- PMID: 35938049
- PMCID: PMC9352529
- DOI: 10.1016/j.isci.2022.104754
The endoplasmic reticulum membrane complex promotes proteostasis of GABAA receptors
Abstract
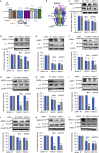
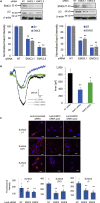
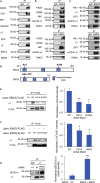
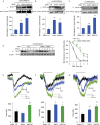
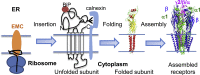
The endoplasmic reticulum membrane complex (EMC) plays a critical role in the biogenesis of tail-anchored proteins and a subset of multi-pass membrane proteins in the endoplasmic reticulum (ER). However, because of nearly exclusive expression of neurotransmitter-gated ion channels in the central nervous system (CNS), the role of the EMC in their biogenesis is not well understood. In this study, we demonstrated that the EMC positively regulates the surface trafficking and thus function of endogenous γ-aminobutyric acid type A (GABAA) receptors, the primary inhibitory ion channels in the mammalian brain. Moreover, among ten EMC subunits, EMC3 and EMC6 have the most prominent effect, and overexpression of EMC3 or EMC6 is sufficient to restore the function of epilepsy-associated GABAA receptor variants. In addition, EMC3 and EMC6 demonstrate endogenous interactions with major neuroreceptors, which depends on their transmembrane domains, suggesting a general role of the EMC in the biogenesis of neuroreceptors.
Keywords: Biological sciences; Cell biology; Molecular biology; Molecular neuroscience; Neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Hsp47 promotes biogenesis of multi-subunit neuroreceptors in the endoplasmic reticulum.Elife. 2024 Jul 4;13:e84798. doi: 10.7554/eLife.84798. Elife. 2024. PMID: 38963323 Free PMC article.
-
The endoplasmic reticulum membrane protein complex subunit Emc6 is essential for rhodopsin localization and photoreceptor cell survival.Genes Dis. 2023 May 23;11(2):1035-1049. doi: 10.1016/j.gendis.2023.03.033. eCollection 2024 Mar. Genes Dis. 2023. PMID: 37692493 Free PMC article.
-
Quantitative interactome proteomics identifies a proteostasis network for GABAA receptors.J Biol Chem. 2022 Oct;298(10):102423. doi: 10.1016/j.jbc.2022.102423. Epub 2022 Aug 27. J Biol Chem. 2022. PMID: 36030824 Free PMC article.
-
The Function, Structure, and Origins of the ER Membrane Protein Complex.Annu Rev Biochem. 2022 Jun 21;91:651-678. doi: 10.1146/annurev-biochem-032620-104553. Epub 2022 Mar 14. Annu Rev Biochem. 2022. PMID: 35287476 Review.
-
The Role of EMC during Membrane Protein Biogenesis.Trends Cell Biol. 2019 May;29(5):371-384. doi: 10.1016/j.tcb.2019.01.007. Epub 2019 Feb 27. Trends Cell Biol. 2019. PMID: 30826214 Review.
Cited by
-
Hsp47 promotes biogenesis of multi-subunit neuroreceptors in the endoplasmic reticulum.Elife. 2024 Jul 4;13:e84798. doi: 10.7554/eLife.84798. Elife. 2024. PMID: 38963323 Free PMC article.
-
EMC rectifies the topology of multipass membrane proteins.Nat Struct Mol Biol. 2024 Jan;31(1):32-41. doi: 10.1038/s41594-023-01120-6. Epub 2023 Nov 13. Nat Struct Mol Biol. 2024. PMID: 37957425 Free PMC article.
-
Pharmacological Analysis of GABAA Receptor and Sigma1R Chaperone Interaction: Research Report I-Investigation of the Anxiolytic, Anticonvulsant and Hypnotic Effects of Allosteric GABAA Receptors' Ligands.Int J Mol Sci. 2023 May 31;24(11):9580. doi: 10.3390/ijms24119580. Int J Mol Sci. 2023. PMID: 37298532 Free PMC article.
-
Pharmacological chaperones restore proteostasis of epilepsy-associated GABAA receptor variants.bioRxiv [Preprint]. 2023 Apr 19:2023.04.18.537383. doi: 10.1101/2023.04.18.537383. bioRxiv. 2023. Update in: Pharmacol Res. 2024 Oct;208:107356. doi: 10.1016/j.phrs.2024.107356. PMID: 37131660 Free PMC article. Updated. Preprint.
-
GABRA1 frameshift variants impair GABAA receptor proteostasis.bioRxiv [Preprint]. 2025 Jan 8:2024.11.28.625971. doi: 10.1101/2024.11.28.625971. bioRxiv. 2025. PMID: 39651292 Free PMC article. Preprint.
References
-
- Absalom N.L., Liao V.W.Y., Johannesen K.M.H., Gardella E., Jacobs J., Lesca G., Gokce-Samar Z., Arzimanoglou A., Zeidler S., Striano P., et al. Gain-of-function and loss-of-function GABRB3 variants lead to distinct clinical phenotypes in patients with developmental and epileptic encephalopathies. Nat. Commun. 2022;13:1822. doi: 10.1038/s41467-022-29280-x. - DOI - PMC - PubMed
-
- Barnes E.M. Assembly and intracellular trafficking of GABA(A) receptors. Int. Rev. Neurobiol. 2001;48:1–29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

