Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells
- PMID: 35938100
- PMCID: PMC9355146
- DOI: 10.3389/fnut.2022.957778
Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts-milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells
Abstract
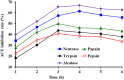
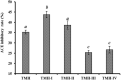
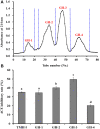
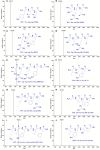
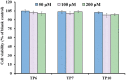
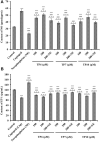
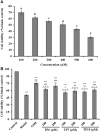
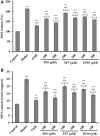
To prepare peptides with high angiotensin-converting enzyme (ACE) inhibitory (ACEi) activity, Alcalase was screened from five proteases and employed to prepare protein hydrolysate (TMH) of skipjack tuna (Katsuwonus pelamis) milts. Subsequently, 10 novel ACEi peptides were isolated from the high-ACEi activity TMH and identified as Tyr-Asp-Asp (YDD), Thr-Arg-Glu (TRE), Arg-Asp-Tyr (RDY), Thr-Glu-Arg-Met (TERM), Asp-Arg-Arg-Tyr-Gly (DRRYG), Ile-Cys-Tyr (ICY), Leu-Ser-Phe-Arg (LSFR), Gly-Val-Arg-Phe (GVRF), Lys-Leu-Tyr-Ala-Leu-Phe (KLYALF), and Ile-Tyr-Ser-Pro (IYSP) with molecular weights of 411.35, 404.41, 452.45, 535.60, 665.69, 397.48, 521.61, 477.55, 753.91, and 478.53 Da, respectively. Among them, the IC50 values of ICY, LSFR, and IYSP on ACE were 0.48, 0.59, and 0.76 mg/mL, respectively. The significant ACEi activity of ICY, LSFR, and IYSP with affinities of -7.0, -8.5, and -8.3 kcal/mol mainly attributed to effectively combining with the ACEi active sites through hydrogen bonding, electrostatic force, and hydrophobic interaction. Moreover, ICY, LSFR, and IYSP could positively influence the production of nitric oxide (NO) and endothelin-1 (ET-1) secretion in human umbilical vein endothelial cells (HUVECs) and weaken the adverse impact of norepinephrine (NE) on the production of NO and ET-1. In addition, ICY, LSFR, and IYSP could provide significant protection to HUVECs against H2O2 damage by increasing antioxidase levels to decrease the contents of reactive oxide species and malondialdehyde. Therefore, the ACEi peptides of ICY, LSFR, and IYSP are beneficial functional molecules for healthy foods against hypertension and cardiovascular diseases.
Keywords: angiotensin-I-converting enzyme (ACE); antihypertensive function; antioxidant activity; milt; peptide; skipjack tuna (Katsuwonus pelamis).
Copyright © 2022 Suo, Zheng, Chi, Luo and Wang.
Figures


Similar articles
-
Preparation, Identification, Molecular Docking Study and Protective Function on HUVECs of Novel ACE Inhibitory Peptides from Protein Hydrolysate of Skipjack Tuna Muscle.Mar Drugs. 2022 Feb 27;20(3):176. doi: 10.3390/md20030176. Mar Drugs. 2022. PMID: 35323475 Free PMC article.
-
Production, identification, in silico analysis, and cytoprotection on H2O2-induced HUVECs of novel angiotensin-I-converting enzyme inhibitory peptides from Skipjack tuna roes.Front Nutr. 2023 Jul 12;10:1197382. doi: 10.3389/fnut.2023.1197382. eCollection 2023. Front Nutr. 2023. PMID: 37502715 Free PMC article.
-
Preparation, Characterization, and Cytoprotective Effects on HUVECs of Fourteen Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides From Protein Hydrolysate of Tuna Processing By-Products.Front Nutr. 2022 Apr 14;9:868681. doi: 10.3389/fnut.2022.868681. eCollection 2022. Front Nutr. 2022. PMID: 35495901 Free PMC article.
-
Antihypertensive peptides derived from egg proteins.J Nutr. 2006 Jun;136(6):1457-60. doi: 10.1093/jn/136.6.1457. J Nutr. 2006. PMID: 16702303 Review.
-
Identifiable Achatina giant neurones: their localizations in ganglia, axonal pathways and pharmacological features.Gen Pharmacol. 1996 Jan;27(1):3-32. doi: 10.1016/0306-3623(95)00113-1. Gen Pharmacol. 1996. PMID: 8742492 Review.
Cited by
-
Study on the antioxidant activity of peptides from soybean meal by fermentation based on the chemical method and AAPH-induced oxidative stress.Food Sci Nutr. 2023 Aug 28;11(10):6634-6647. doi: 10.1002/fsn3.3612. eCollection 2023 Oct. Food Sci Nutr. 2023. PMID: 37823157 Free PMC article.
-
Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from the Collagens of Monkfish (Lophius litulon) Swim Bladders: Isolation, Characterization, Molecular Docking Analysis and Activity Evaluation.Mar Drugs. 2023 Sep 28;21(10):516. doi: 10.3390/md21100516. Mar Drugs. 2023. PMID: 37888451 Free PMC article.
-
Chemical Characterization of Honeysuckle Polyphenols and Their Alleviating Function on Ultraviolet B-Damaged HaCaT Cells by Modulating the Nrf2/NF-κB Signaling Pathways.Antioxidants (Basel). 2024 Feb 28;13(3):294. doi: 10.3390/antiox13030294. Antioxidants (Basel). 2024. PMID: 38539828 Free PMC article.
-
Systematical Investigation on Anti-Fatigue Function and Underlying Mechanism of High Fischer Ratio Oligopeptides from Antarctic Krill on Exercise-Induced Fatigue in Mice.Mar Drugs. 2024 Jul 19;22(7):322. doi: 10.3390/md22070322. Mar Drugs. 2024. PMID: 39057431 Free PMC article.
-
Enzymatic Hydrolysis Systems Enhance the Efficiency and Biological Properties of Hydrolysates from Frozen Fish Processing Co-Products.Mar Drugs. 2024 Dec 28;23(1):14. doi: 10.3390/md23010014. Mar Drugs. 2024. PMID: 39852515 Free PMC article.
References
-
- Auwal SM, Zainal Abidin N, Zarei M, Tan CP, Saari N. Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS ONE. (2019) 14:e0197644. 10.1371/journal.pone.0197644 - DOI - PMC - PubMed
-
- Suo SK, Zhao YQ, Wang YM, Pan XY, Chi CF, Wang B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from protein hydrolysate of Mytilus edulis: isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. (2022). 10.1039/D2FO00275B (In Press) - DOI - PubMed
-
- Abdelhedi O, Nasri M. Basic and recent advances in marine antihypertensive peptides: production, structure-activity relationship and bioavailability. Trends Food Sci Technol. (2019) 88:543–57. 10.1016/j.tifs.2019.04.002 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

