Association of gut microbiota with sort-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: A cross-sectional, non-controlled study
- PMID: 35938126
- PMCID: PMC9355148
- DOI: 10.3389/fnut.2022.930626
Association of gut microbiota with sort-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: A cross-sectional, non-controlled study
Abstract
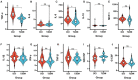
Emerging evidence suggests that gut microbiota, short-chain fatty acids (SCFAs), and inflammatory cytokines play important roles in the pathogenesis of diabetic cognitive impairment (DCI). However, little is known about alterations of gut microbiota and SCFA levels as well as the relationships between inflammatory cytokines and cognitive function in Chinese DCI patients. Herein, the differences in the gut microbiota, plasma SCFAs, and inflammatory cytokines in DCI patients and type 2 diabetes mellitus (T2DM) patients were explored. A cross-sectional study of 30 DCI patients and 30 T2DM patients without mild cognitive impairment (MCI) was conducted in Tianjin city, China. The gut microbiota, plasma SCFAs, and inflammatory cytokines were determined using 16S ribosomal RNA (rRNA) gene sequencing, gas chromatography-mass spectrometry (GC-MS), and Luminex immunofluorescence assays, respectively. In addition, the correlation between gut microbiota and DCI clinical characteristics, SCFAs, and inflammatory cytokines was investigated. According to the results, at the genus level, DCI patients presented a greater abundance of Gemmiger, Bacteroides, Roseburia, Prevotella, and Bifidobacterium and a poorer abundance of Escherichia and Akkermansia than T2DM patients. The plasma concentrations of acetic acid, propionic acid, isobutyric acid, and butyric acid plummeted in DCI patients compared to those in T2DM patients. TNF-α and IL-8 concentrations in plasma were significantly higher in DCI patients than in T2DM patients. Moreover, the concentrations of acetic acid, propionic acid, butyric acid, and isovaleric acid in plasma were negatively correlated with TNF-α, while those of acetic acid and butyric acid were negatively correlated with IL-8. Furthermore, the abundance of the genus Alloprevotella was negatively correlated with butyric acid, while that of Holdemanella was negatively correlated with propanoic acid and isobutyric acid. Fusobacterium abundance was negatively correlated with propanoic acid. Clostridium XlVb abundance was negatively correlated with TNF-α, while Shuttleworthia abundance was positively correlated with TNF-α. It was demonstrated that the gut microbiota alterations were accompanied by a change in SCFAs and inflammatory cytokines in DCI in Chinese patients, potentially causing DCI development. These findings might help to identify more effective microbiota-based therapies for DCI in the future.
Keywords: 16S rRNA gene; gut microbiota; inflammatory cytokines; mild cognitive impairment; short-chain fatty acids; type 2 diabetes mellitus.
Copyright © 2022 Du, Li, An, Song and Lu.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

