Genomic and Immunological Characterization of Pyroptosis in Lung Adenocarcinoma
- PMID: 35938142
- PMCID: PMC9348947
- DOI: 10.1155/2022/6905588
Genomic and Immunological Characterization of Pyroptosis in Lung Adenocarcinoma
Abstract
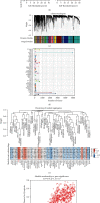
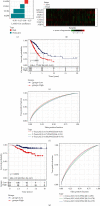
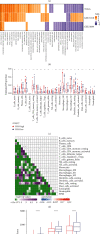
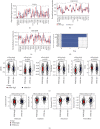
Pyroptosis is a programmed cell death that may either promote or hinder cancer growth under different circumstances. Pyroptosis-related genes (PRGs) could be a useful target for cancer therapy, and are uncommon in lung adenocarcinoma (LUAD). The expression profiles, mutation data and clinical information of LUAD patients were included in this study. A pyroptosis-related prognostic risk score (PPRS) model was constructed by performing Cox regression, weighted gene co-expression network analysis (WGCNA), and least absolute shrinkage and selection operator (LASSO) analysis to score LUAD patients. Somatic mutation and copy number variation (CNV), tumor immunity, and sensitivity to immunotherapy/chemotherapy were compared between different PPRS groups. Clinical parameters of LUAD were combined with PPRS to construct a decision tree and nomogram. Red module was highly positively correlated with pyroptosis. Seven genes (FCRLB, COTL1, GNG10, CASP4, DOK1, CCR2, and AQP8) were screened from the red module to construct a PPRS model. Significantly lower overall survival (OS), higher incidence of somatic mutation and CNV, elevated infiltration level of the immune cell together with increased probability of immune escape were observed in LUAD patients with higher PPRS, and were more sensitive to Cisplatin, Docetaxel, and Vinorelbine. We constructed a new PPRS model for patients with LUAD. The model might have clinical significance in the prediction of the prognosis of patients with LUAD and in the efficacy of chemotherapy and immunotherapy.
Copyright © 2022 Yaobo Song et al.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

