Single-Cell Transcriptomics of Proliferative Phase Endometrium: Systems Analysis of Cell-Cell Communication Network Using CellChat
- PMID: 35938159
- PMCID: PMC9352955
- DOI: 10.3389/fcell.2022.919731
Single-Cell Transcriptomics of Proliferative Phase Endometrium: Systems Analysis of Cell-Cell Communication Network Using CellChat
Abstract
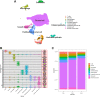
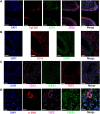
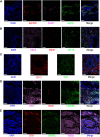
The endometrium thickness increases by which endometrial angiogenesis occurs in parallel with the rapid growth of endometrium during the proliferative phase, which is orchestrated by complex cell-cell interactions and cytokine networks. However, the intercellular communication has not been fully delineated. In the present work, we studied the cell-cell interactome among cells of human proliferative phase endometrium using single-cell transcriptomics. The transcriptomes of 33,240 primary endometrial cells were profiled at single-cell resolution. CellChat was used to infer the cell-cell interactome by assessing the gene expression of receptor-ligand pairs across cell types. In total, nine cell types and 88 functionally related signaling pathways were found. Among them, growth factors and angiogenic factor signaling pathways, including EGF, FGF, IGF, PDGF, TGFb, VEGF, ANGPT, and ANGPTL that are highly associated with endometrial growth, were further analyzed and verified. The results showed that stromal cells and proliferating stromal cells represented cell-cell interaction hubs with a large number of EGF, PDGF incoming signals, and FGF outgoing signals. Endothelial cells exhibited cell-cell interaction hubs with a plenty of VEGF, TGFb incoming signals, and ANGPT outgoing signals. Unciliated epithelial cells, ciliated epithelial cells, and macrophages exhibited cell-cell interaction hubs with substantial EGF outgoing signals. Ciliated epithelial cells represented cell-cell interaction hubs with a large number of IGF and TGFb incoming signals. Smooth muscle cells represented lots of PDGF incoming signals and ANGPT and ANGPTL outgoing signals. This study deconvoluted complex intercellular communications at the single-cell level and predicted meaningful biological discoveries, which deepened the understanding of communications among endometrial cells.
Keywords: angiogenesis; cell communication network; endometrium; proliferation; single-cell sequencing.
Copyright © 2022 Fang, Tian, Sui, Guo, Hu, Lai, Liao, Li, Feng, Jin and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

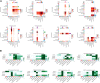


References
LinkOut - more resources
Full Text Sources

