The Anti-Leukemia Effect of Ascorbic Acid: From the Pro-Oxidant Potential to the Epigenetic Role in Acute Myeloid Leukemia
- PMID: 35938170
- PMCID: PMC9352950
- DOI: 10.3389/fcell.2022.930205
The Anti-Leukemia Effect of Ascorbic Acid: From the Pro-Oxidant Potential to the Epigenetic Role in Acute Myeloid Leukemia
Abstract
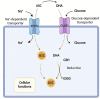
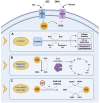
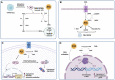
Data derived from high-throughput sequencing technologies have allowed a deeper understanding of the molecular landscape of Acute Myeloid Leukemia (AML), paving the way for the development of novel therapeutic options, with a higher efficacy and a lower toxicity than conventional chemotherapy. In the antileukemia drug development scenario, ascorbic acid, a natural compound also known as Vitamin C, has emerged for its potential anti-proliferative and pro-apoptotic activities on leukemic cells. However, the role of ascorbic acid (vitamin C) in the treatment of AML has been debated for decades. Mechanistic insight into its role in many biological processes and, especially, in epigenetic regulation has provided the rationale for the use of this agent as a novel anti-leukemia therapy in AML. Acting as a co-factor for 2-oxoglutarate-dependent dioxygenases (2-OGDDs), ascorbic acid is involved in the epigenetic regulations through the control of TET (ten-eleven translocation) enzymes, epigenetic master regulators with a critical role in aberrant hematopoiesis and leukemogenesis. In line with this discovery, great interest has been emerging for the clinical testing of this drug targeting leukemia epigenome. Besides its role in epigenetics, ascorbic acid is also a pivotal regulator of many physiological processes in human, particularly in the antioxidant cellular response, being able to scavenge reactive oxygen species (ROS) to prevent DNA damage and other effects involved in cancer transformation. Thus, for this wide spectrum of biological activities, ascorbic acid possesses some pharmacologic properties attractive for anti-leukemia therapy. The present review outlines the evidence and mechanism of ascorbic acid in leukemogenesis and its therapeutic potential in AML. With the growing evidence derived from the literature on situations in which the use of ascorbate may be beneficial in vitro and in vivo, we will finally discuss how these insights could be included into the rational design of future clinical trials.
Keywords: acute myeloid leukemia; ascorbic acid; epigenetic regulation; oxidative stress; vitamin C.
Copyright © 2022 Travaglini, Gurnari, Antonelli, Silvestrini, Noguera, Ottone and Voso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Aberrant DNA Methylation in Acute Myeloid Leukemia and Its Clinical Implications.Int J Mol Sci. 2019 Sep 16;20(18):4576. doi: 10.3390/ijms20184576. Int J Mol Sci. 2019. PMID: 31527484 Free PMC article. Review.
-
New promising developments for potential therapeutic applications of high-dose ascorbate as an anticancer drug.Hematol Oncol Stem Cell Ther. 2021 Sep;14(3):179-191. doi: 10.1016/j.hemonc.2020.11.002. Epub 2020 Nov 24. Hematol Oncol Stem Cell Ther. 2021. PMID: 33278349 Review.
-
Pharmacological GLUT3 salvage augments the efficacy of vitamin C-induced TET2 restoration in acute myeloid leukemia.Leukemia. 2023 Aug;37(8):1638-1648. doi: 10.1038/s41375-023-01954-5. Epub 2023 Jul 1. Leukemia. 2023. PMID: 37393342
-
Epigenetic Therapies for Acute Myeloid Leukemia and Their Immune-Related Effects.Front Cell Dev Biol. 2019 Oct 11;7:207. doi: 10.3389/fcell.2019.00207. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31681756 Free PMC article. Review.
-
The role of vitamin C in epigenetic cancer therapy.Free Radic Biol Med. 2021 Jul;170:179-193. doi: 10.1016/j.freeradbiomed.2021.03.017. Epub 2021 Mar 28. Free Radic Biol Med. 2021. PMID: 33789122 Review.
Cited by
-
Targeting chemoresistance and mitochondria-dependent metabolic reprogramming in acute myeloid leukemia.Front Oncol. 2023 Sep 7;13:1244280. doi: 10.3389/fonc.2023.1244280. eCollection 2023. Front Oncol. 2023. PMID: 37746249 Free PMC article. Review.
-
Role of reactive oxygen species in myelodysplastic syndromes.Cell Mol Biol Lett. 2024 Apr 14;29(1):53. doi: 10.1186/s11658-024-00570-0. Cell Mol Biol Lett. 2024. PMID: 38616283 Free PMC article. Review.
-
Ex Vivo Anti-Leukemic Effect of Exosome-like Grapefruit-Derived Nanovesicles from Organic Farming-The Potential Role of Ascorbic Acid.Int J Mol Sci. 2023 Oct 27;24(21):15663. doi: 10.3390/ijms242115663. Int J Mol Sci. 2023. PMID: 37958646 Free PMC article.
-
Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2.Antioxidants (Basel). 2023 Oct 30;12(11):1929. doi: 10.3390/antiox12111929. Antioxidants (Basel). 2023. PMID: 38001782 Free PMC article.
-
Therapeutic innovations: targeting ROS production in AML with natural and synthetic compounds.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 31. doi: 10.1007/s00210-025-04054-6. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40163149 Review.
References
-
- Aldoss I., Mark L., Vrona J., Ramezani L., Weitz I., Mohrbacher A. M., et al. (2014). Adding Ascorbic Acid to Arsenic Trioxide Produces Limited Benefit in Patients with Acute Myeloid Leukemia Excluding Acute Promyelocytic Leukemia. Ann. Hematol. 93, 1839–1843. 10.1007/s00277-014-2124-y - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

