Effects of ultra-low dose hormone therapy on biochemical bone turnover markers in postmenopausal women: A randomized, placebo-controlled, double-blind trial
- PMID: 35938207
- PMCID: PMC9500172
- DOI: 10.1177/20533691221116769
Effects of ultra-low dose hormone therapy on biochemical bone turnover markers in postmenopausal women: A randomized, placebo-controlled, double-blind trial
Abstract
Objective: Evaluate the effects of ultra-low-dose hormone therapy (Ultra-LD HT) with 17β-estradiol 0.5 mg and norethisterone acetate 0.1 mg (E2 0.5/NETA 0.1) versus placebo on bone turnover markers (BTM) in postmenopausal women.
Study design: A multicenter, double-blind, randomized, placebo-controlled study was performed with 107 participants who received one tablet daily of E2 0.5/NETA 0.1 or placebo for 24-weeks. Bone formation markers-N-terminal propeptide of type I procollagen (PINP) and Bone-specific alkaline phosphatase (BSAP), and bone resorption markers-C-telopeptide of type I collagen (CTX-I) and N-telopeptide crosslinked of type I collagen (NTX) were assessed before and at 12 and 24-weeks of treatment.
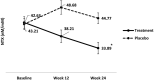
Results: Women treated with E2 0.5/NETA 0.1 had a significant reduction in the PINP marker from baseline (58.49 ± 21.12 μg/L) to week 12 (48.31 ± 20.99 μg/L) and week 24 (39.16 ± 16.50 μg/L). Placebo group, the PINP marker did not differ significantly. The analysis of the BSAP indicated a significant increase in the placebo group (13.8 ± 5.09 μg/L and 16.29 ± 4.3 μg/L, at baseline and week 24, respectively), whereas in the treatment group the values did not change. The analysis of the NTX marker showed a significant reduction only in the treatment group (43.21 ± 15.26 nM/mM and 33.89 ± 14.9 nM/mM, at baseline and week 24, respectively). CTX-I had a significant decrease in the treatment group from baseline (0.3 ± 0.16 ng/L) to week 12 (0.21 ± 0.14 ng/L) and week 24 (0.21 ± 0.12 ng/L).
Conclusion: Women receiving E2 0.5/NETA 0.1 experienced reductions in bone resorption and formation markers, an expected effect during the anti-resorptive therapy, suggesting a protective bone effect with the Ultra-LD HT.
Keywords: bone mineral density; bone turnover marker; estrogen; menopause; ultra-low-dose hormone therapy.
Conflict of interest statement
Figures




Similar articles
-
A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women.Osteoporos Int. 2000;11(2):177-87. doi: 10.1007/pl00004180. Osteoporos Int. 2000. PMID: 10793878 Clinical Trial.
-
Effects of intranasal 17beta-estradiol on bone turnover and serum insulin-like growth factor I in postmenopausal women.J Clin Endocrinol Metab. 1999 Jul;84(7):2390-7. doi: 10.1210/jcem.84.7.5848. J Clin Endocrinol Metab. 1999. PMID: 10404809 Clinical Trial.
-
Effect of low-dose transdermal E2/NETA on the reduction of postmenopausal bone loss in women.Menopause. 2003 May-Jun;10(3):241-9. doi: 10.1097/00042192-200310030-00012. Menopause. 2003. PMID: 12792297 Clinical Trial.
-
Treatment-Related Changes in Bone Turnover and Fracture Risk Reduction in Clinical Trials of Anti-Resorptive Drugs: A Meta-Regression.J Bone Miner Res. 2018 Apr;33(4):634-642. doi: 10.1002/jbmr.3355. Epub 2018 Jan 10. J Bone Miner Res. 2018. PMID: 29318649
-
The effect of statins on bone turnover biomarkers: a systematic review and meta-analysis of randomized controlled trials.Endocr J. 2023 May 29;70(5):473-480. doi: 10.1507/endocrj.EJ22-0512. Epub 2023 Mar 15. Endocr J. 2023. PMID: 36928061
Cited by
-
Alendronate sodium demonstrates significant clinical advantages in treating osteoporosis secondary to severe fractures.Am J Transl Res. 2025 Jun 15;17(6):4516-4523. doi: 10.62347/MFPV8667. eCollection 2025. Am J Transl Res. 2025. PMID: 40672612 Free PMC article.
-
Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women.Pharmaceuticals (Basel). 2025 Jan 8;18(1):61. doi: 10.3390/ph18010061. Pharmaceuticals (Basel). 2025. PMID: 39861124 Free PMC article. Review.
-
Effects of isoflavone interventions on bone turnover markers and factors regulating bone metabolism in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials.Arch Osteoporos. 2024 Dec 21;20(1):2. doi: 10.1007/s11657-024-01467-3. Arch Osteoporos. 2024. PMID: 39708251
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

