Cardiac and respiratory muscle responses to dietary N-acetylcysteine in rats consuming a high-saturated fat, high-sucrose diet
- PMID: 35938289
- PMCID: PMC9633399
- DOI: 10.1113/EP090332
Cardiac and respiratory muscle responses to dietary N-acetylcysteine in rats consuming a high-saturated fat, high-sucrose diet
Abstract
New findings: What is the central question of this study? This study addresses whether a high-fat, high-sucrose diet causes cardiac and diaphragm muscle abnormalities in male rats and whether supplementation with the antioxidant N-acetylcysteine reverses diet-induced dysfunction. What is the main finding and its importance? N-Acetylcysteine attenuated the effects of high-fat, high-sucrose diet on markers of cardiac hypertrophy and diastolic dysfunction, but neither high-fat, high-sucrose diet nor N-acetylcysteine affected the diaphragm. These results support the use of N-acetylcysteine to attenuate cardiovascular dysfunction induced by a 'Western' diet.
Abstract: Individuals with overweight or obesity display respiratory and cardiovascular dysfunction, and oxidative stress is a causative factor in the general aetiology of obesity and of skeletal and cardiac muscle pathology. Thus, this preclinical study aimed to define diaphragmatic and cardiac morphological and functional alterations in response to an obesogenic diet in rats and the therapeutic potential of an antioxidant supplement, N-acetylcysteine (NAC). Young male Wistar rats consumed ad libitum a 'lean' or high-saturated fat, high-sucrose (HFHS) diet for ∼22 weeks and were randomized to control or NAC (2 mg/ml in the drinking water) for the last 8 weeks of the dietary intervention. We then evaluated diaphragmatic and cardiac morphology and function. Neither HFHS diet nor NAC supplementation affected diaphragm-specific force, peak power or morphology. Right ventricular weight normalized to estimated body surface area, left ventricular fractional shortening and posterior wall maximal shortening velocity were higher in HFHS compared with lean control animals and not restored by NAC. In HFHS rats, the elevated deceleration rate of early transmitral diastolic velocity was prevented by NAC. Our data showed that the HFHS diet did not compromise diaphragmatic muscle morphology or in vitro function, suggesting other possible contributors to breathing abnormalities in obesity (e.g., abnormalities of neuromuscular transmission). However, the HFHS diet resulted in cardiac functional and morphological changes suggestive of hypercontractility and diastolic dysfunction. Supplementation with NAC did not affect diaphragm morphology or function but attenuated some of the cardiac abnormalities in the rats receiving the HFHS diet.
Keywords: diaphragm; diastolic function; hypertrophy; oxidants.
© 2022 The Authors. Experimental Physiology © 2022 The Physiological Society.
Conflict of interest statement
Author Disclosure Statement
The authors declare no conflict of interest.
Figures






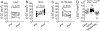
References
-
- Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, Ezquer M & Cabello-Verrugio C. (2016). High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxid Med Cell Longev 2016, 9047821. - PMC - PubMed
-
- Allwood MA, Foster AJ, Arkell AM, Beaudoin MS, Snook LA, Romanova N, Murrant CL, Holloway GP, Wright DC & Simpson JA. (2015). Respiratory muscle weakness in the Zucker diabetic fatty rat. Am J Physiol Regul Integr Comp Physiol 309, R780–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

