Recent advances in nanotechnology-enabled biosensors for detection of exosomes as new cancer liquid biopsy
- PMID: 35938477
- PMCID: PMC9837302
- DOI: 10.1177/15353702221110813
Recent advances in nanotechnology-enabled biosensors for detection of exosomes as new cancer liquid biopsy
Abstract
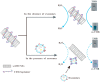
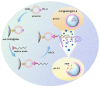
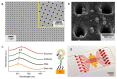
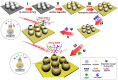
Cancer liquid biopsy detects circulating biomarkers in body fluids, provides information that complements medical imaging and tissue biopsy, allows sequential monitoring of cancer development, and, therefore, has shown great promise in cancer screening, diagnosis, and prognosis. Exosomes (also known as small extracellular vesicles) are cell-secreted, nanosized vesicles that transport biomolecules such as proteins and RNAs for intercellular communication. Exosomes are actively involved in cancer development and progression and have become promising circulating biomarkers for cancer liquid biopsy. Conventional exosome characterization methods such as quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) are limited by low sensitivity, tedious process, large sample volume, and high cost. To overcome these challenges, new biosensors have been developed to offer sensitive, simple, fast, high throughput, low sample consumption, and cost-effective detection of exosomal biomarkers. In this review, we summarized recent advances in nanotechnology-enabled biosensors that detect exosomal RNAs (both microRNAs and mRNAs) and proteins for cancer screening, diagnosis, and prognosis. The biosensors were grouped based on their sensing mechanisms, including fluorescence-based biosensors, colorimetric biosensors, electrical/electrochemical biosensors, plasmonics-based biosensors, surface-enhanced Raman spectroscopy (SERS)-based biosensors, and inductively coupled plasma mass spectrometry (ICP-MS) and photothermal biosensors. The future directions for the development of exosome-based biosensors were discussed.
Keywords: Exosomes; biosensors; cancer liquid biopsy; extracellular vesicles; mRNA; microRNA; protein.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Recent Advances in Exosomal Protein Detection Via Liquid Biopsy Biosensors for Cancer Screening, Diagnosis, and Prognosis.AAPS J. 2018 Mar 8;20(2):41. doi: 10.1208/s12248-018-0201-1. AAPS J. 2018. PMID: 29520676 Free PMC article. Review.
-
Recent advances in nanomaterial-based biosensors for the detection of exosomes.Anal Bioanal Chem. 2021 Jan;413(1):83-102. doi: 10.1007/s00216-020-03000-0. Epub 2020 Nov 8. Anal Bioanal Chem. 2021. PMID: 33164151 Review.
-
The new advance of exosome-based liquid biopsy for cancer diagnosis.J Nanobiotechnology. 2024 Oct 8;22(1):610. doi: 10.1186/s12951-024-02863-0. J Nanobiotechnology. 2024. PMID: 39380060 Free PMC article. Review.
-
Recent Advances in Biosensors for Detecting Cancer-Derived Exosomes.Trends Biotechnol. 2019 Nov;37(11):1236-1254. doi: 10.1016/j.tibtech.2019.04.008. Trends Biotechnol. 2019. PMID: 31104858 Review.
-
Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview.Biosens Bioelectron. 2020 Aug 1;161:112222. doi: 10.1016/j.bios.2020.112222. Epub 2020 Apr 21. Biosens Bioelectron. 2020. PMID: 32365010 Review.
Cited by
-
Recent Advances in Fluorescence Resonance Energy Transfer (FRET) Biosensors for Exosomes.Curr Issues Mol Biol. 2025 Mar 28;47(4):235. doi: 10.3390/cimb47040235. Curr Issues Mol Biol. 2025. PMID: 40699635 Free PMC article. Review.
-
Liquid Biopsy in the Clinical Management of Cancers.Int J Mol Sci. 2024 Aug 6;25(16):8594. doi: 10.3390/ijms25168594. Int J Mol Sci. 2024. PMID: 39201281 Free PMC article. Review.
-
The therapeutic bionanoscience interface.Exp Biol Med (Maywood). 2022 Dec;247(23):2065-2066. doi: 10.1177/15353702221144090. Epub 2022 Dec 19. Exp Biol Med (Maywood). 2022. PMID: 36533612 Free PMC article. No abstract available.
-
Exosomes as Powerful Biomarkers in Cancer: Recent Advances in Isolation and Detection Techniques.Int J Nanomedicine. 2024 Feb 26;19:1923-1949. doi: 10.2147/IJN.S453545. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38435755 Free PMC article. Review.
-
Potential Immunohistochemical Biomarkers for Grading Oral Dysplasia: A Literature Review.Biomedicines. 2024 Mar 5;12(3):577. doi: 10.3390/biomedicines12030577. Biomedicines. 2024. PMID: 38540190 Free PMC article. Review.
References
-
- Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic—implementation issues and future challenges. Nat Rev Clin Oncol 2021; 18:297–312 - PubMed
-
- Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531–48 - PubMed
-
- Möller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer 2020;20:697–709 - PubMed
-
- LeBleu VS, Kalluri R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 2020;6:767–74 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

