Long-term effects of Pfizer-BioNTech COVID-19 vaccinations on platelets
- PMID: 35938513
- PMCID: PMC9538905
- DOI: 10.1002/cyto.a.24677
Long-term effects of Pfizer-BioNTech COVID-19 vaccinations on platelets
Abstract
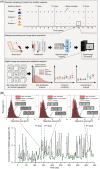
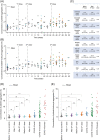
There is a global concern about the safety of COVID-19 vaccines associated with platelet function. However, their long-term effects on overall platelet activity remain poorly understood. Here we address this problem by image-based single-cell profiling and temporal monitoring of circulating platelet aggregates in the blood of healthy human subjects, before and after they received multiple Pfizer-BioNTech (BNT162b2) vaccine doses over a time span of nearly 1 year. Results show no significant or persisting platelet aggregation trends following the vaccine doses, indicating that any effects of vaccinations on platelet turnover, platelet activation, platelet aggregation, and platelet-leukocyte interaction was insignificant.
Keywords: COVID-19; mRNA vaccine; platelet aggregation; thrombosis.
© 2022 International Society for Advancement of Cytometry.
Conflict of interest statement
Nao Nitta and Keisuke Goda are shareholders of CYBO.
Figures
Comment in
-
Effects of COVID-19 vaccinations on platelets: Correspondence.Cytometry A. 2023 Apr;103(4):353. doi: 10.1002/cyto.a.24689. Epub 2022 Sep 16. Cytometry A. 2023. PMID: 36076341 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

