Automated slice-specific z-shimming for functional magnetic resonance imaging of the human spinal cord
- PMID: 35938527
- PMCID: PMC9704784
- DOI: 10.1002/hbm.26018
Automated slice-specific z-shimming for functional magnetic resonance imaging of the human spinal cord
Abstract
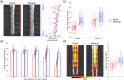
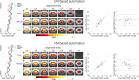
Functional magnetic resonance imaging (fMRI) of the human spinal cord faces many challenges, such as signal loss due to local magnetic field inhomogeneities. This issue can be addressed with slice-specific z-shimming, which compensates for the dephasing effect of the inhomogeneities using a slice-specific gradient pulse. Here, we aim to address outstanding issues regarding this technique by evaluating its effects on several aspects that are directly relevant for spinal fMRI and by developing two automated procedures in order to improve upon the time-consuming and subjective nature of manual selection of z-shims: one procedure finds the z-shim that maximizes signal intensity in each slice of an EPI reference-scan and the other finds the through-slice field inhomogeneity for each EPI-slice in field map data and calculates the required compensation gradient moment. We demonstrate that the beneficial effects of z-shimming are apparent across different echo times, hold true for both the dorsal and ventral horn, and are also apparent in the temporal signal-to-noise ratio (tSNR) of EPI time-series data. Both of our automated approaches were faster than the manual approach, lead to significant improvements in gray matter tSNR compared to no z-shimming and resulted in beneficial effects that were stable across time. While the field-map-based approach performed slightly worse than the manual approach, the EPI-based approach performed as well as the manual one and was furthermore validated on an external corticospinal data-set (N > 100). Together, automated z-shimming may improve the data quality of future spinal fMRI studies and lead to increased reproducibility in longitudinal studies.
Keywords: automated z-shim; functional magnetic resonance imaging; magnetic field inhomogeneities; signal loss; spinal cord; temporal signal-to-noise ratio.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The Max Planck Institute for Human Cognitive and Brain Sciences has an institutional research agreement with Siemens Healthcare. Nikolaus Weiskopf holds a patent on acquisition of MRI data during spoiler gradients (US 10,401,453 B2). Nikolaus Weiskopf was a speaker at an event organized by Siemens Healthcare and was reimbursed for the travel expenses.
Figures


Similar articles
-
Impact of through-slice gradient optimization for dynamic slice-wise shimming in the cervico-thoracic spinal cord.Magn Reson Med. 2025 Sep;94(3):1090-1102. doi: 10.1002/mrm.30543. Epub 2025 May 1. Magn Reson Med. 2025. PMID: 40312894 Free PMC article.
-
A second-order and slice-specific linear shimming technique to improve spinal cord fMRI.Magn Reson Imaging. 2023 Oct;102:151-163. doi: 10.1016/j.mri.2023.06.012. Epub 2023 Jun 21. Magn Reson Imaging. 2023. PMID: 37353180
-
Single, slice-specific z-shim gradient pulses improve T2*-weighted imaging of the spinal cord.Neuroimage. 2012 Feb 1;59(3):2307-15. doi: 10.1016/j.neuroimage.2011.09.038. Epub 2011 Sep 22. Neuroimage. 2012. PMID: 21979381
-
In vivo B0 field shimming methods for MRI at 7T.Neuroimage. 2018 Mar;168:71-87. doi: 10.1016/j.neuroimage.2017.06.013. Epub 2017 Jun 7. Neuroimage. 2018. PMID: 28602943 Free PMC article. Review.
-
Spinal cord MRI at 7T.Neuroimage. 2018 Mar;168:437-451. doi: 10.1016/j.neuroimage.2017.07.003. Epub 2017 Jul 3. Neuroimage. 2018. PMID: 28684332 Free PMC article. Review.
Cited by
-
Reliability of task-based fMRI in the dorsal horn of the human spinal cord.bioRxiv [Preprint]. 2024 Jun 25:2023.12.22.572825. doi: 10.1101/2023.12.22.572825. bioRxiv. 2024. Update in: Imaging Neurosci (Camb). 2024 Aug 22;2:imag-2-00273. doi: 10.1162/imag_a_00273. PMID: 38187724 Free PMC article. Updated. Preprint.
-
Impact of through-slice gradient optimization for dynamic slice-wise shimming in the cervico-thoracic spinal cord.Magn Reson Med. 2025 Sep;94(3):1090-1102. doi: 10.1002/mrm.30543. Epub 2025 May 1. Magn Reson Med. 2025. PMID: 40312894 Free PMC article.
-
Spatial distribution of hand-grasp motor task activity in spinal cord functional magnetic resonance imaging.Hum Brain Mapp. 2023 Dec 1;44(17):5567-5581. doi: 10.1002/hbm.26458. Epub 2023 Aug 22. Hum Brain Mapp. 2023. PMID: 37608682 Free PMC article.
-
Harnessing the power of endogenous pain control mechanisms for novel therapeutics: how might innovations in neuroimaging help?Curr Opin Support Palliat Care. 2023 Sep 1;17(3):150-155. doi: 10.1097/SPC.0000000000000653. Epub 2023 Jun 22. Curr Opin Support Palliat Care. 2023. PMID: 37352611 Free PMC article. Review.
-
Shimming toolbox: An open-source software toolbox for B0 and B1 shimming in MRI.Magn Reson Med. 2023 Apr;89(4):1401-1417. doi: 10.1002/mrm.29528. Epub 2022 Nov 28. Magn Reson Med. 2023. PMID: 36441743 Free PMC article.
References
-
- Barry, R. L. , Conrad, B. N. , Maki, S. , Watchmaker, J. M. , McKeithan, L. J. , Box, B. A. , Weinberg, Q. R. , Smith, S. A. , & Gore, J. C. (2021). Multi‐shot acquisitions for stimulus‐evoked spinal cord BOLD fMRI. Magnetic Resonance in Medicine, 85(4), 2016–2026. 10.1002/mrm.28570 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

