Molecular Epidemiology of Mycobacterium abscessus Isolates Recovered from German Cystic Fibrosis Patients
- PMID: 35938728
- PMCID: PMC9431180
- DOI: 10.1128/spectrum.01714-22
Molecular Epidemiology of Mycobacterium abscessus Isolates Recovered from German Cystic Fibrosis Patients
Abstract
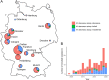
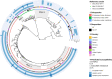
Infections due to Mycobacterium abscessus are a major cause of mortality and morbidity in cystic fibrosis (CF) patients. Furthermore, M. abscessus has been suspected to be involved in person-to-person transmissions. In 2016, dominant global clonal complexes (DCCs) that occur worldwide among CF patients have been described. To elucidate the epidemiological situation of M. abscessus among CF patients in Germany and to put these data into a global context, we performed whole-genome sequencing of a set of 154 M. abscessus isolates from 123 German patients treated in 14 CF centers. We used MTBseq pipeline to identify clusters of closely related isolates and correlate those with global findings. Genotypic drug susceptibility for macrolides and aminoglycosides was assessed by characterization of the erm(41), rrl, and rrs genes. By this approach, we could identify representatives of all major DCCs (Absc 1, Absc 2, and Mass 1) in our cohort. Intrapersonal isolates showed higher genetic relatedness than interpersonal isolates (median 3 SNPs versus 16 SNPs; P < 0.001). We further identified four clusters with German patients from same centers clustering with less than 25 SNPs distance (range 3 to 18 SNPs) but did not find any hint for in-hospital person-to-person transmission. This is the largest study investigating phylogenetic relations of M. abscessus isolates in Germany. We identified representatives of all reported DCCs but evidence for nosocomial transmission remained inconclusive. Thus, the occurrence of genetically closely related isolates of M. abscessus has to be interpreted with care, as a direct interhuman transmission cannot be directly deduced. IMPORTANCE Mycobacterium abscessus is a major respiratory pathogen in cystic fibrosis (CF) patients. Recently it has been shown that dominant global clonal complexes (DCCs) have spread worldwide among CF patients. This study investigated the epidemiological situation of M. abscessus among CF patients in Germany by performing whole-genome sequencing (WGS) of a set of 154 M. abscessus from 123 German patients treated in 14 CF centers. This is the largest study investigating the phylogenetic relationship of M. abscessus CF isolates in Germany.
Keywords: German CF registry; Mycobacterium abscessus; cystic fibrosis; dominant circulating clones; hospital transmission; nontuberculous mycobacteria; whole-genome sequencing.
Conflict of interest statement
The authors declare a conflict of interest. N. Wetzstein has nothing to disclose. M. Diricks reports institutional funding (Research Center Borstel) from the German Research Foundation (DFG) under Germanys Excellence Strategy EXC 2167 (Precision Medicine in Inflammation), the Leibniz Science Campus Evolutionary Medicine of the LUNG (EvoLUNG), and the German Center for Infection Research (DZIF). T. A. Kohl has nothing to disclose. T. A. Wichelhaus reports research grants from MSD and Deutsche Krebshilfe, honoraria for lectures and consulting from Insmed and Osartis. S. Andres has nothing to disclose. L. Paulowski has nothing to disclose. C. Schwarz reports grants from Vertex Pharamaceuticals and the German Mykoviszidose Institut gGmbH and the German Federal Ministry of Health, honoraria for lectures and presentations from Vertex Pharamaceuticals, Chiesi GmbH and AbbVie Deutschland. A. Lewin has nothing to disclose. J. Kehrman has nothing to disclose. B. C. Kahl reports honoraria for lectures and presentations and supports for attending meetings and/or travel from Pfizer Germany. K. Dichtl has nothing to disclose. C. Hügel reports Grants from the German Mykoviszidose Institut gGmbH. O. Eickmeier has nothing to disclose. C. Smaczny has nothing to disclose. A. Schmidt has nothing to disclose. S. Zimmerman reports Grants from Bosch HealthCare Systems and on the MSD Investigator-initiated grant PNEU-MISP. L. Nährlich reports institutional fees for study participation by the German Center for Lung Research; Vertex Pharamaceuticals and Boehringer Ingelheim; participation Trial Steering Committee for CF STORM and duties as Medical lead of the German CF-registry and Pharmacovigilance study manager of the ECFSPR. S. Hafkemeyer has nothing to disclose. S. Niemann reports grants from the German Research Foundation (DFG) under the Excellence Cluster EXC 2167 (Precision Medicine in Inflammation) and the Leibniz Science Campus Evolutionary Medicine of the LUNG (EvoLUNG). F. P. Maurer reports grants from Mukoviszidose Institut gGmbH, Bonn and the German Federal Ministry of Health. M. Hogardt reports grants from Mukoviszidose Institut gGmbH and the German Federal Ministry of Health, honoraria for lectures from the Landesärztekammer Hessen and bioMérieux, and funding from Merck AG outside the submitted work.
Figures


References
-
- Schnabel D, Gaines J, Nguyen DB, Esposito DH, Ridpath A, Yacisin K, Poy JA, Mullins J, Burns R, Lijewski V, McElroy NP, Ahmad N, Harrison C, Parinelli EJ, Beaudoin AL, Posivak-Khouly L, Pritchard S, Jensen BJ, Toney NC, Moulton-Meissner HA, Nyangoma EN, Barry AM, Feldman KA, Blythe D, Perz JF, Morgan OW, Kozarsky P, Brunette GW, Sotir M, Centers for Disease Control and Prevention (CDC) . 2014. Notes from the field: rapidly growing nontuberculous Mycobacterium wound infections among medical tourists undergoing cosmetic surgeries in the Dominican Republic–multiple states, March 2013–February 2014. MMWR Morb Mortal Wkly Rep 63:201–202. http://www.ncbi.nlm.nih.gov/pubmed/24598597 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

