The Glycine-Glucolipid of Alcanivorax borkumensis Is Resident to the Bacterial Cell Wall
- PMID: 35938787
- PMCID: PMC9397105
- DOI: 10.1128/aem.01126-22
The Glycine-Glucolipid of Alcanivorax borkumensis Is Resident to the Bacterial Cell Wall
Abstract
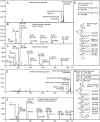
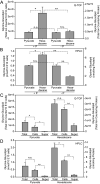
The marine bacterium Alcanivorax borkumensis produces a surface-active glycine-glucolipid during growth with long-chain alkanes. A high-performance liquid chromatography (HPLC) method was developed for absolute quantification. This method is based on the conversion of the glycine-glucolipid to phenacyl esters with subsequent measurement by HPLC with diode array detection (HPLC-DAD). Different molecular species were separated by HPLC and identified as glucosyl-tetra(3-hydroxy-acyl)-glycine with varying numbers of 3-hydroxy-decanoic acid or 3-hydroxy-octanoic acid groups via mass spectrometry. The growth rate of A. borkumensis cells with pyruvate as the sole carbon source was elevated compared to hexadecane as recorded by the increase in cell density as well as oxygen/carbon dioxide transfer rates. The amount of the glycine-glucolipid produced per cell during growth on hexadecane was higher compared with growth on pyruvate. The glycine-glucolipid from pyruvate-grown cells contained considerable amounts of 3-hydroxy-octanoic acid, in contrast to hexadecane-grown cells, which almost exclusively incorporated 3-hydroxy-decanoic acid into the glycine-glucolipid. The predominant proportion of the glycine-glucolipid was found in the cell pellet, while only minute amounts were present in the cell-free supernatant. The glycine-glucolipid isolated from the bacterial cell broth, cell pellet, or cell-free supernatant showed the same structure containing a glycine residue, in contrast to previous reports, which suggested that a glycine-free form of the glucolipid exists which is secreted into the supernatant. In conclusion, the glycine-glucolipid of A. borkumensis is resident to the cell wall and enables the bacterium to bind and solubilize alkanes at the lipid-water interface. IMPORTANCE Alcanivorax borkumensis is one of the most abundant marine bacteria found in areas of oil spills, where it degrades alkanes. The production of a glycine-glucolipid is considered an essential element for alkane degradation. We developed a quantitative method and determined the structure of the A. borkumensis glycine-glucolipid in different fractions of the cultures after growth in various media. Our results show that the amount of the glycine-glucolipid in the cells by far exceeds the amount measured in the supernatant, confirming the proposed cell wall localization. These results support the scenario that the surface hydrophobicity of A. borkumensis cells increases by producing the glycine-glucolipid, allowing the cells to attach to the alkane-water interface and form a biofilm. We found no evidence for a glycine-free form of the glucolipid.
Keywords: 3-hydroxy fatty acid; Alcanivorax borkumensis; HPLC; biosurfactants; glucolipid; glucose; glycine; mass spectrometry; oil spill.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Identification and quantification of biosurfactants produced by the marine bacterium Alcanivorax borkumensis by hyphenated techniques.Anal Bioanal Chem. 2023 Dec;415(29-30):7067-7084. doi: 10.1007/s00216-023-04972-5. Epub 2023 Oct 11. Anal Bioanal Chem. 2023. PMID: 37819435 Free PMC article.
-
Biosurfactant biosynthesis by Alcanivorax borkumensis and its role in oil biodegradation.Nat Chem Biol. 2025 May 9. doi: 10.1038/s41589-025-01908-1. Online ahead of print. Nat Chem Biol. 2025. PMID: 40346253
-
Adaptation of the hydrocarbonoclastic bacterium Alcanivorax borkumensis SK2 to alkanes and toxic organic compounds: a physiological and transcriptomic approach.Appl Environ Microbiol. 2013 Jul;79(14):4282-93. doi: 10.1128/AEM.00694-13. Epub 2013 May 3. Appl Environ Microbiol. 2013. PMID: 23645199 Free PMC article.
-
Genome sequence completed of Alcanivorax borkumensis, a hydrocarbon-degrading bacterium that plays a global role in oil removal from marine systems.J Biotechnol. 2003 Dec 19;106(2-3):215-20. doi: 10.1016/j.jbiotec.2003.07.013. J Biotechnol. 2003. PMID: 14651863 Review.
-
Occurrence, production, and export of lipophilic compounds by hydrocarbonoclastic marine bacteria and their potential use to produce bulk chemicals from hydrocarbons.Appl Microbiol Biotechnol. 2010 May;86(6):1693-706. doi: 10.1007/s00253-010-2515-5. Epub 2010 Mar 31. Appl Microbiol Biotechnol. 2010. PMID: 20354694 Review.
Cited by
-
High-quality physiology of Alcanivorax borkumensis SK2 producing glycolipids enables efficient stirred-tank bioreactor cultivation.Front Bioeng Biotechnol. 2023 Nov 23;11:1325019. doi: 10.3389/fbioe.2023.1325019. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38084272 Free PMC article.
-
Deciphering the biosynthesis of a bacterial detergent for cleanup of marine oil spills.Nat Chem Biol. 2025 May 21. doi: 10.1038/s41589-025-01933-0. Online ahead of print. Nat Chem Biol. 2025. PMID: 40399585 No abstract available.
-
Antibacterial Ingredients and Modes of the Methanol-Phase Extract from the Fruit of Amomum villosum Lour.Plants (Basel). 2024 Mar 14;13(6):834. doi: 10.3390/plants13060834. Plants (Basel). 2024. PMID: 38592864 Free PMC article.
-
Identification of Antibacterial Components and Modes in the Methanol-Phase Extract from a Herbal Plant Potentilla kleiniana Wight et Arn.Foods. 2023 Apr 13;12(8):1640. doi: 10.3390/foods12081640. Foods. 2023. PMID: 37107435 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

