Analytical Sensitivity of Eight Different SARS-CoV-2 Antigen-Detecting Rapid Tests for Omicron-BA.1 Variant
- PMID: 35938792
- PMCID: PMC9430749
- DOI: 10.1128/spectrum.00853-22
Analytical Sensitivity of Eight Different SARS-CoV-2 Antigen-Detecting Rapid Tests for Omicron-BA.1 Variant
Abstract
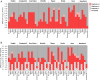
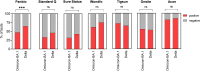
The emergence of each novel SARS-CoV-2 variant of concern (VOC) requires investigation of its potential impact on the performance of diagnostic tests in use, including antigen-detecting rapid diagnostic tests (Ag-RDTs). Although anecdotal reports have been circulating that the newly emerged Omicron-BA.1 variant is in principle detectable by Ag-RDTs, few data on sensitivity are available. We have performed (i) analytical sensitivity testing with cultured virus in eight Ag-RDTs and (ii) retrospective testing in duplicates with clinical samples from vaccinated individuals with Omicron-BA.1 (n = 59) or Delta (n = 54) breakthrough infection on seven Ag-RDTs. Overall, in our analytical study we have found heterogenicity between Ag-RDTs for detecting Omicron-BA.1. When using cultured virus, we observed a trend toward lower endpoint sensitivity for Omicron-BA.1 detection than for earlier circulating SARS-CoV-2 and the other VOCs. In our retrospective study, the detection of Delta and Omicron-BA.1 was assessed in a comparable set of stored clinical samples using seven Ag-RDTs. Four hundred ninety-seven of all 826 tests (60.17%) performed on Omicron-BA.1 samples were positive, compared to 489/756 (64.68%) for Delta samples. In the analytical study, the sensitivity for both Omicron-BA.1 and Delta between the Ag-RDTs was variable. All seven Ag-RDTs showed comparable sensitivities to detect Omicron-BA.1 and Delta in the retrospective study. IMPORTANCE Sensitivity for detecting Omicron-BA.1 shows high heterogenicity between Ag-RDTs, necessitating a careful consideration when using these tests to guide infection prevention measures. Analytical and retrospective testing is a proxy and timely solution to generate rapid performance data, but it is not a replacement for clinical evaluations, which are urgently needed. Biological and technical reasons for detection failure by some Ag-RDTs need to be further investigated.
Keywords: COVID-19; Omicron variant; Omicron-BA.1 variant; SARS-CoV-2; antigen-detecting rapid diagnostic tests; variants of concern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization. 2020. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos.... Accessed 28 April 2021.
-
- World Health Organization. 2021. Weekly epidemiological update on COVID-19. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- World Health Organization. 2022. Tracking SARS-CoV-2 variants. World Health Organization, Geneva, Switzerland. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 8 December 2021.
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

