Quorum Sensing Regulation of a Major Facilitator Superfamily Transporter Affects Multiple Streptococcal Virulence Factors
- PMID: 35938850
- PMCID: PMC9487453
- DOI: 10.1128/jb.00176-22
Quorum Sensing Regulation of a Major Facilitator Superfamily Transporter Affects Multiple Streptococcal Virulence Factors
Abstract
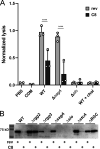
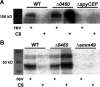
Cell-cell signaling mediated by Rgg-family transcription factors and their cognate pheromones is conserved in Firmicutes, including all streptococci. In Streptococcus pyogenes, or group A strep (GAS), one of these systems, the Rgg2/3 quorum sensing (QS) system, has been shown to regulate phenotypes, including cellular aggregation and biofilm formation, lysozyme resistance, and macrophage immunosuppression. Here, we show the abundance of several secreted virulence factors (streptolysin O, SpyCEP, and M protein) decreases upon induction of QS. The main mechanism underlying the changes in protein levels appears to be transcriptional, occurs downstream of the QS circuit, and is dysregulated by the deletion of an Rgg2/3 QS-regulated major facilitator superfamily (MFS) transporter. Additionally, we identify this MFS transporter as the factor responsible for a previously observed increase in aminoglycoside sensitivity in QS-induced cells. IMPORTANCE The production of virulence factors is a tightly regulated process in bacterial pathogens. Efforts to elucidate the mechanisms by which genes are regulated may advance the understanding of factors influencing pathogen behavior or cellular physiology. This work finds expression of a major facilitator superfamily (MFS) transporter, which is governed by a quorum sensing (QS) system, impacts the expression of multiple virulence factors and accounts for QS-dependent antibiotic susceptibility. Although the mechanism underlying this effect is not clear, MFS orthologs with high sequence similarity from S. pneumoniae and S. porcinus were unable to substitute indicating substrate specificity of the GAS MFS gene. These findings demonstrate novel associations between expression of a transmembrane transporter and virulence factor expression and aminoglycoside transport.
Keywords: M protein; SpyCEP; Streptococcus pyogenes; major facilitator superfamily transporter; streptolysin O.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antagonistic Rgg regulators mediate quorum sensing via competitive DNA binding in Streptococcus pyogenes.mBio. 2013 Jan 2;3(6):e00333-12. doi: 10.1128/mBio.00333-12. mBio. 2013. PMID: 23188510 Free PMC article.
-
A novel chemical inducer of Streptococcus quorum sensing acts by inhibiting the pheromone-degrading endopeptidase PepO.J Biol Chem. 2018 Jan 19;293(3):931-940. doi: 10.1074/jbc.M117.810994. Epub 2017 Dec 4. J Biol Chem. 2018. PMID: 29203527 Free PMC article.
-
Identification of Quorum-Sensing Inhibitors Disrupting Signaling between Rgg and Short Hydrophobic Peptides in Streptococci.mBio. 2015 May 12;6(3):e00393-15. doi: 10.1128/mBio.00393-15. mBio. 2015. PMID: 25968646 Free PMC article.
-
Quorum sensing: A less known mode of communication among fungi.Microbiol Res. 2018 May;210:51-58. doi: 10.1016/j.micres.2018.03.007. Epub 2018 Mar 21. Microbiol Res. 2018. PMID: 29625658 Review.
-
Mechanisms of quorum sensing and strategies for quorum sensing disruption in aquaculture pathogens.J Fish Dis. 2015 Sep;38(9):771-86. doi: 10.1111/jfd.12299. Epub 2014 Sep 15. J Fish Dis. 2015. PMID: 25219871 Review.
Cited by
-
Identifying genetic determinants of Streptococcus pyogenes-host interactions in a murine intact skin infection model.Cell Rep. 2023 Nov 28;42(11):113332. doi: 10.1016/j.celrep.2023.113332. Epub 2023 Oct 26. Cell Rep. 2023. PMID: 37889753 Free PMC article.
-
Metronidazole response profiles of Gardnerella species are congruent with phylogenetic and comparative genomic analyses.Genome Med. 2025 Mar 25;17(1):28. doi: 10.1186/s13073-025-01446-4. Genome Med. 2025. PMID: 40133961 Free PMC article.
-
Nosocomial transmission of tet(x3), bla NDM-1 and bla OXA-97-carrying Acinetobacter baumannii conferring resistance to eravacycline and omadacycline, the Netherlands, March to August 2021.Euro Surveill. 2024 Jul;29(28):2400019. doi: 10.2807/1560-7917.ES.2024.29.28.2400019. Euro Surveill. 2024. PMID: 38994602 Free PMC article.
-
The Proteomic and Transcriptomic Landscapes Altered by Rgg2/3 Activity in Streptococcus pyogenes.J Bacteriol. 2022 Nov 15;204(11):e0017522. doi: 10.1128/jb.00175-22. Epub 2022 Oct 31. J Bacteriol. 2022. PMID: 36314832 Free PMC article.
References
-
- Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. 2017. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 377:713–722. 10.1056/NEJMoa1603693. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

