Synthesis and Biological Evaluation of Bicyclo[1.1.1]pentane-Containing Aromatic Lipoxin A4 Analogues
- PMID: 35938947
- PMCID: PMC9400386
- DOI: 10.1021/acs.orglett.2c02345
Synthesis and Biological Evaluation of Bicyclo[1.1.1]pentane-Containing Aromatic Lipoxin A4 Analogues
Abstract
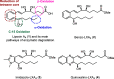
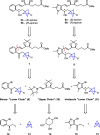
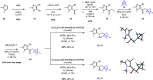
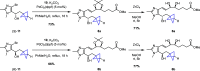
Lipoxins are important drivers of inflammation resolution, suggesting a potential therapeutic benefit. Bicyclo[1.1.1]pentanes (BCPs) are potential isosteric replacements for arenes and/or alkyl groups within drug candidates. We carried out an asymmetric synthesis of four BCP-containing synthetic lipoxin A4 mimetics (BCP-sLXms) in which the key steps were a Suzuki coupling, an asymmetric ketone reduction, and a triethylborane-initiated radical bicyclopentylation. These mimetics were screened for their impact on inflammatory responses, and one imidazolo-BCP-sLXm (6a) was found to possess high anti-inflammatory activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Cicchese J. M.; Evans S.; Hult C.; Joslyn L. R.; Wessler T.; Millar J. A.; Marino S.; Cilfone N. A.; Mattila J. T.; Linderman J. J.; Kirschner D. E. Dynamic Balance of Pro- and Anti-Inflammatory Signals Controls Disease and Limits Pathology. Immunol. Rev. 2018, 285 (1), 147–167. 10.1111/imr.12671. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

